The advantages of stenting for treatment of coarctation of the aorta compared to balloon angioplasty is that it avoids excessive dilation of the adjacent aorta and elastic recoil of the vessel, in addition to sealing potential acute dissections, thereby reducing the incidence of aneurysms, recurrence of coarctation and aortic rupture. Most hospitals consider it the procedure of choice for native coarctation and for recurrent coarctation in patients with weights greater than 25 to 30 kg, in whom the calibre of femoral arteries is usually adequate.1 Below this weight, although there is not sufficient evidence to establish the optimal therapeutic approach, angioplasty or surgery are used in most cases.2,3
We conducted a retrospective analysis of all the patients aged less than 18 years managed with percutaneous stent implantation between 1996 and 2020 and followed up for at least 1 year. The resulting consecutive sample included 25 patients with a mean age of 10.6 years and a mean weight of 33.9 kg, of who 16% had weights of less than 30 kg. Thirteen patients were treated for native coarctation and 12 for recurrence of coarctation treated with surgery. Three of the cases of recurrent coarctation had required angioplasty due to a previous recurrence before placement of the stent. The most frequent associated cardiac defects were bicuspid aortic valve and ventricular septal defect (VSD) (Table 1).
Clinical and haemodynamic characteristics of the sample.
| Clinical variables | |
| Mean age ± SD (years) | 10.6 ± 4.6 |
| Mean weight ± SD (kg) | 33.9 ± 18.9 |
| Male sex | 18 (72%) |
| Associated heart defects (other than aortic valve defect) | 5 (20%) |
| VSD | |
| Anomalous pulmonary venous return | 1 (4%) |
| Bicuspid aortic valve | 14 (56%) |
| Significant aortic valve disease | 2 (8%) |
| Type of coarctation | |
| Native | 13 (52%) |
| Recurrent | 12 (48%) |
| Previous surgical treatment | |
| End-to-end anastomosis | 8 (32%) |
| Subclavian flap | 3 (12%) |
| Patch aortoplasty | 1 (4%) |
| Previous angioplasty | 3 (12%) |
| Haemodynamic variables | |
| Aortic arch diameter ± SD (mm) | 13.2 ± 3.8 |
| Diaphragmatic aorta diameter ± SD (mm) | 15.12 ± 4.4 |
| Coarctation diameter ± SD (mm) | |
| Previous procedure ± SD | 5.1 ± 2.2 |
| After stenting ± SD | 14.6 ± 3.7 |
| Transaortic gradient (invasive) | |
| Previous ± SD | 33.6 ± 14.3 |
| After stenting ± SD | 2.8 ± 4.1 |
| Stent diameter ± SD (mm) | 15 ± 4.2 |
| Stent length ± SD (mm) | 38.8 ± 18.2 |
| Need of more than 1 stent | 2 (8%) |
| ePTFE-covered stent | 6 (24%) |
ePTFE: expanded polytetrafluoroethylene; SD, standard deviation; VSD, ventricular septal defect.
In 20 cases (80%) the coarctation was at the level of the aortic isthmus, in 2 (12%) at the level of the aortic arch (8%) at the level of the diaphragmatic aorta.
All procedures were performed via the femoral approach with guiding catheters ranging from 8 F to 16 F under general anaesthesia. The size of the stent was chosen based on the measurements obtained by computed tomography and angiography, with a 1:1 ratio for the stent diameter and the diameter of the aorta at the level of the diaphragm unless there was a significant difference in diameter at the level of the aortic arch, which requires adjusting the stent to the proximal diameter for post-dilation of the distal end with a larger balloon to adjust it to the size of the diaphragmatic aorta.
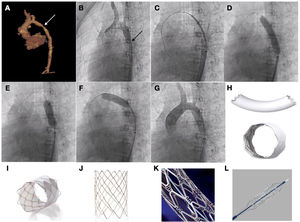
Stenting was successful in 100% of cases, achieving both a reduction of the transcoarctation pressure gradient and an increase in the diameter of the aortic lumen, differences that were statistically significant. In 19 cases, the implanted stents were uncovered: Palmaz (Cardinal Health Inc, Dublin, OH, USA), CP stent (NuMED Inc, Hopkinton, NY, USA) and Formula (Cook Medical, Bloomington, IN, USA). Another 6 were covered with expanded polytetrafluoroethylene: covered CP stent (NuMED Inc, Hopkinton, NY, USA) and BeGraft (Bentley InnoMed GmbH, Hechingen, Germany) (Fig. 1).
Patient aged 10 years with Williams syndrome and recurrent coarctation of aorta referred for percutaneous stent implantation. (A and B) CT and angiography images showing narrowing of the aortic lumen. (C) Advancing the stent to the target area. (D) Inflation of stent with visualization of reduced expansion at the level of the isthmus. (E and F) Post-dilation of both the distal and the proximal portions of the stent. (G) Angiographic verification. (H) BeGraft stent. (I) Covered CP stent. (J) CP stent (uncovered). (K) Palmaz Genesis stent. (L) Formula stent.
All patients remained in follow-up for a mean of 12.9 years (SD, ±7.4), with monitoring of symptoms and radiological features. Five patients (20%) required reintervention in mean of 6.9 ± 8.7 years. In 2 patients it consisted of surgical repair with prosthetic material due to a calibre mismatch, in 2 cases of implantation of a secondary stent overlapping the first due to recurrence of coarctation at the distal end, and in 1 case in redilation of the proximal end of the stent. The observed complications were 2 cases of femoral artery thrombosis that resolved with conservative management with anticoagulants.
The variables that were significantly associated with the need of reintervention were previous repair with balloon angioplasty and coarctation at the level of the aortic arch or the diaphragm (P < 0.05). One patient (4%) developed postcardiotomy shock after undergoing a hybrid procedure for surgical repair of a complex cardiac defect and stenting at the level of the aortic arch, and died 24 hours after.
In the paediatric and adolescent population, the cardiac anatomy is usually not complex or associated with calcification or substantial tortuosity, so the procedure is usually performed with uncovered stents, which have a very favourable safety profile in both the short and long term, with an incidence of complications at the level of the aortic wall of less than 2%, and a smaller calibre, which is important for the prevention of complications at the vascular access site. Covered stents are reserved for cases of complex cardiac anatomy, presence of prostheses or Turner or Williams syndrome, which are associated with a more fragile aortic wall and therefore a higher risk of complications.4,5 It is important to select the appropriate time for the intervention and the correct stent size, since growth can give rise to a calibre mismatch, and consequently stents should be selected to allow further expansion with growth, even considering sequential dilation strategies, which has an impact on the rate of reintervention compared to the adult population.6 Taking these aspects into account, we consider that stent implantation for management of coarctation of aorta in the paediatric and adolescent population is safe and effective in the long term.
Please cite this article as: Fernández González L, Alcibar Villa J, Blanco Mata R, Arriola Meabe J, Galdeano Miranda JM. Experiencia unicéntrica en el tratamiento percutáneo con stent de la coartación de aorta en niños y adolescentes. An Pediatr (Barc). 2022;96:542–544.