The objective of the study was to establish the normal range for the levels of antithrombin (AT), protein C (PC), and protein S (PS) in the first week post birth in mother–infant pairings, adjusting for obstetric and perinatal factors, based on 2 different laboratory methods.
MethodsDeterminations were carried out in 83 healthy term neonates and their mothers, establishing 3 postpartum age groups: 1−2 days, 3 days, and 4−7 days.
ResultsThere were no differences in the levels of any of the proteins between the different age groups in neonates or mothers in the first week post birth. The adjusted analysis found no association with obstetric or perinatal factors. The AT and PC levels were higher in mothers compared to infants (P < .001), while the PS levels were similar in both. Overall, the correlation of maternal and infant protein values was poor, except for the levels of free PS in the first 2 days after delivery. Although we found no differences based on which of the 2 laboratory methods was applied, the absolute values did differ.
El objetivo fue establecer valores de normalidad de antitrombina (AT), la proteína C (PC) y la proteína S (PS) dentro de la primera semana después del nacimiento en el binomio madre-recién nacido, ajustados por factores obstétricos y perinatales, según dos métodos de laboratorio diferentes.
MétodosSe realizaron determinaciones en 83 neonatos a término sanos y sus madres, con tres grupos de edad posparto: días 1–2, 3 y 4–7.
ResultadosNo hubo diferencias para ninguna de las proteínas en los distintos grupos de edad de los neonatos y las madres dentro de la primera semana posparto. El análisis ajustado no mostró ninguna asociación con factores obstétricos o perinatales. Los valores de AT y PC en las madres fueron mayores que en los neonatos (P < 0,001), mientras que la PS mostró valores similares. La correlación global de los valores entre los pares madre-recién nacido fue escasa, salvo para la PS libre en los en los siguientes dos días al parto. Aunque no se encontraron diferencias entre los dos métodos de laboratorio, los valores absolutos fueron diferentes.
The diagnosis of a clotting disorder during the puerperium poses a challenge in clinical practice, as significant haemostatic changes occur in both mother and newborn during this period.1–3 However, very few studies have sought to establish normal haemostatic values in mothers and infants during the perinatal period.1,4,5 In addition, it is not known whether obstetric and perinatal characteristics have an impact on measured values or whether there is an association between anticoagulant protein changes in mothers and in infants in the postpartum period.
Moreover, it is recommended that each laboratory define age-dependent coagulation reference ranges based on their testing equipment and technique, as measured values are highly dependent on the applied method.6,7
The aim of our study was to establish normal values for antithrombin (AT), protein C (PC), and protein S (PS) in the first week post birth in mother–infant pairings adjusted for obstetric and perinatal factors using 2 different laboratory methods.
Materials and methodsHealthy term neonate and mother pairings with no family history of coagulation disorders were recruited in the maternity wards of 2 tertiary care hospitals. Vitamin K was administered to the infants in adherence to the standard of care. The study was approved by the research ethics committees of the participating hospitals in the framework of a broader project that included research into the role of thrombophilia risk factors in the aetiology and pathogenesis of perinatal stroke (PI08/1366). We obtained informed consent from the parents of the neonates and the mothers themselves.
The blood samples were obtained at the time of sample collection for routine newborn metabolic screening. Samples of 1 mL were added to 3% buffered sodium citrate, at a ratio of 1 part citrate to 9 parts blood. Plasma was obtained by centrifugation at 3500 rpm at 4 °C for 10 min. Values of AT, PC, and PS were measured with 2 different techniques and reagents, and expressed as percentages (Supplementary Table S1).
Statistical analysisWe summarised qualitative data as absolute and relative frequencies, and quantitative data with the median and interquartile range (IQR) or the mean and standard deviation (SD) depending on their distribution. We compared normally distributed continuous variables with the Student t test or analysis of variance (ANOVA), and non-normally distributed quantitative variables with the Mann–Whitney U test or the Kruskal–Wallis test as applicable. Categorical (dichotomous) variables were compared using the χ2 test or Fisher exact test.
We conducted multivariate analyses to assess the association of perinatal acidosis, gestational age, infant sex, and small for gestational age with the coagulation protein values of the neonate and the type of delivery, age and maternal body mass index. We calculated the Spearman correlation coefficient (rs) to establish the strength and direction of the association between mother and infant protein values.
All reported P values are 2-sided, and we considered P values of less than 0.05 statistically significant. The statistical analysis was carried out with the software SPSS, version 25 (IBM, Armonk, NY, USA) and Stata version 15.1 (StataCorp, College Station, TX, USA).
Results88 healthy neonates born during the study period and their mothers were invited to participate. Four families rejected participation, and we recruited another 84 mother–infant pairings as controls. One family was excluded due to hyperhomocysteinaemia. The main characteristics of the mother–infant pairings are summarised in Supplementary Table S2.
Table 1 presents the values of the 3 anticoagulant proteins measured in each of the hospitals with their respective laboratory methods. We did not find differences between the protein values of infants and mothers in the first week post birth based on age group in the raw analysis or adjusting for confounding variables in the mother or infant. We did not find differences between the 2 laboratory methods.
Reference values of antithrombin, protein C, and protein S (%) for neonates and their mothers during the postpartum period.
| Hospital A (N = 52)a | Hospital B (N = 31)b | ||||||
|---|---|---|---|---|---|---|---|
| Days 1−2 | Day 3 | Days 4−7 | Total (Days 1−3) | Days 1−2 | Day 3 | Total (Days 1−3) | |
| INFANTS | |||||||
| Antithrombin, n | 23 | 14 | 13 | 37 | 23 | 6 | 29 |
| Mean | 62.3 | 60.9 | 61.6 | 61.7 | 60.3 | 66.3 | 60.3 |
| Median | 60.4 | 61.3 | 59.3 | 61.1 | 60 | 66.5 | 60 |
| 95% CI | 44−109 | 53−76 | 48−78 | 44−109 | 47−72 | 42−75 | 42−75 |
| Protein C, n | 23 | 14 | 13 | 37 | 23 | 6 | 29 |
| Mean | 41.1 | 41.4 | 35.7 | 41.2 | 34.7 | 38.2 | 35.4 |
| Median | 37 | 36.7 | 35.7 | 36.9 | 33 | 41 | 33 |
| 95% CI | 28−125 | 24−68 | 28−44 | 24−125 | 14−54 | 13−54 | 13−54 |
| Free protein S, n | 23 | 14 | 13 | 37 | NA | NA | NA |
| Mean | 42.4 | 39.6 | 33.7 | 41.4 | |||
| Median | 35.9 | 38.1 | 31.5 | 35.9 | |||
| 95% CI | 17−129 | 25−61 | 6.0−61 | 17−129 | |||
| Total protein S, n | NA | NA | NA | NA | 21 | 6 | 27 |
| Mean | 48 | 54.7 | 50.5 | ||||
| Median | 49.3 | 57.7 | 50 | ||||
| 95% CI | 35−71 | 43−63 | 35−71 | ||||
| Mothers | |||||||
| Antithrombin, n | 22 | 15 | 15 | 37 | 24 | 6 | 30 |
| Mean | 100.7 | 99.8 | 106.4 | 100.4 | 102.3 | 108.8 | 103.6 |
| Median | 99.4 | 98.1 | 104 | 98.6 | 102 | 109 | 104 |
| 95% CI | 67−144 | 87−117 | 78−143 | 67−144 | 81−134 | 90−123 | 81−134 |
| Protein C, n | 22 | 15 | 15 | 37 | 24 | 6 | 30 |
| Mean | 116.2 | 120.5 | 117.0 | 117.9 | 126.0 | 135.2 | 127.9 |
| Median | 114.7 | 119.1 | 119.1 | 115.9 | 128 | 134 | 128.5 |
| 95% CI | 74−152 | 95−149 | 72−143 | 74−152 | 90−166 | 105−176 | 90−176 |
| Free protein S, n | 22 | 15 | 15 | 37 | NA | NA | NA |
| Mean | 42.6 | 44.8 | 48.2 | 43.5 | |||
| Median | 39.5 | 46 | 56.8 | 42.3 | |||
| 95% CI | 19−78 | 23−66 | 22−86 | 19−78 | |||
| Total protein S, n | NA | NA | NA | NA | 24 | 6 | 30 |
| Mean | 52.1 | 59.5 | 53.6 | ||||
| Median | 54 | 51.5 | 54 | ||||
| 95% CI | 29−77 | 39−93 | 29−93 | ||||
CI: confidence interval; IQR: interquartile range; NA: not applicable. Mean, median and boundaries including 95% of the population are shown for each assay. n denotes the number of individual samples for each group.
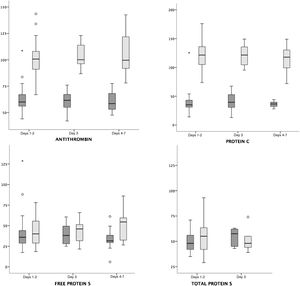
The AT and PC values were higher in mothers compared to infants in every age groups (P < .001). The total protein S values were similar in mothers and infants, and we only found a tendency toward higher values in mothers compared to infants in the free PS on days 4–7 post birth (P = .06) (Fig. 1, Table 1).
Comparison of maternal and neonatal values of antithrombin, protein C, and protein S in mother–infant payrings.
Legend: Median and interquartile range of maternal (light grey) and neonatal (dark grey) protein values (%). Antithrombin (hospital A + B) and protein C (hospital A + B) values were higher in mothers compared to infants in all age groups (P < .001), while free protein S (hospital A) and total protein S (hospital B) values were similar in mothers and infants.
The correlation analysis showed a poor association between maternal and neonatal values of AT, PC, and PS, with an rs of 0.157 (P = .16), 0.268 (P = .016) and 0.374 (P = .001), respectively. The strongest association between mother and infant values was found in free protein S values in the 1-to-2 days post birth group, with a moderate correlation (rs, 0.626; P = .001) (Supplementary Table S3).
DiscussionHaemostatic balance is an evolving process, as the haemostatic system changes and matures continuously from foetal to adult life.1 Although 3 previous studies produced values measured in the first week of life, they made no formal comparisons between different ages and did not analyse the influence of maternal or infant characteristics.1,4,5 We believe that our results are relevant, especially for haematologists, paediatricians and obstetricians, as questions come up in clinical practice concerning whether values of these proteins change after delivery in the mother or infant and whether abnormal values in the either patient could be indicative of disorders that should be ruled out or monitored in the other.
Previous studies have suggested an association between neonatal levels of these proteins and perinatal acidosis8 or small for gestational age.9 However, we did not find any associations between the measured values and perinatal factors, including the sex of the infant.
Throughout our study we confirmed maternal levels of AT and PC were higher than neonatal levels in the first days post birth. Meanwhile, the levels of PS remained similar, probably due to a marked decrease in maternal levels after delivery compared to during pregnancy.2,3 On the other hand, although anticoagulant proteins do not cross the placental barrier,10 we explored whether there was a relationship between maternal values and those of their offspring. It has been hypothesized that there could be common molecular signals or signalling pathways in response to a stressful situation, such as the peripartum, that could modify or influence both environments (maternal and neonatal) in parallel.5 This may explain the moderate correlation that we found between mother and infant free PS values in the first 2 days post birth, but not afterwards, when changes in the infant can be explained by the maturation of the haemostatic system that starts once the foetus is separated from the placenta. However, we found a poor correlation between mother–infant values for the remaining proteins. It would be interesting to explore how the correlation may be affected in the presence of diseases associated with marked changes in these proteins in the mother.
In pregnant women, it has been well established that the risk for thromboembolism rises shortly after birth, largely because the haemostatic balance moves toward hypercoagulability during pregnancy.11 In fact, the haemostasis of pregnancy and the puerperium has been found to differ from that of nonpregnant women.2,3 We did not compare postpartum anticoagulant protein values with values outside the puerperium period; nevertheless, we found no changes in the values measured in the first week following delivery, nor in the association of these values with obstetric factors such as type of delivery, age, and body mass index. We found only one study that measured these proteins at days 1 and 2 post birth, but it did not formally compare the values.2
Previous studies have demonstrated that reference ranges for coagulation assays are analyser- and reagent-dependent.6 Although we found no differences between the 2 laboratory methods, the absolute values differed between the 2 hospitals and compared to other published studies,3,5 corroborating the described variability associated with different analysers and reagents. It is worth noting that a poor understanding of these differences in absolute values can lead to diagnosing disorders that patients do not really have and to therapeutic interventions that are not actually indicated.6 In addition, the diagnosis of AT, PC, and PS deficiencies during the neonatal period may be misleading, as only very low or undetectable levels in this age group support the diagnosis of a heterozygous deficiency.1,4,12
The main limitation of our study is that we did not reach the recommended 30 samples for each protein for most of the age groups required to achieve accurate normal reference values.13
In conclusion, according to our study, values remain stable for the first week post birth in both mothers and their infants; and specific laboratory ranges for age should be used to avoid overdiagnosis and missed diagnosis.
FundingThis study was funded by the Instituto de Salud Carlos III (PI08/1366) and the European Regional Development Fund.
Conflicts of interestThe authors have no conflicts of interest to disclose.
Author contributionsMaria Garrido collected the data, performed the literature search and wrote the manuscript. Juan Arnaez and Alfredo García-Alix participated in the design of the study, carried out research, and contributed to the drafting of the manuscript. Ana Martín-Ancel participated in the research and reviewed the manuscript. Hermenegildo González contributed to the interpretation of the data and reviewed the manuscript. All authors approved the final manuscript.