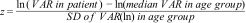The acid-labile subunit (ALS) plays an important role in the endocrine effects of insulin-like growth factors (IGFs) on target tissues. Historically, it has attracted limited attention. The aim of our study was to describe the normal range of ALS in healthy children and its association with other growth factors.
Patients and methodsWe designed a cross-sectional descriptive study. We collected data on age, height, body mass index, gestational age, anthropometry at birth and serum levels of ALS, IGF1 and IGFBP3 in healthy children aged 2–15 years with a normal height. The levels of ALS, IGF1 and IGFBP3 were measured by ELISA. We fitted GAMLSS normalization models to standardize the variables.
ResultsSamples were collected from 446 children. In prepubertal children, the levels of ALS, IGF1 and IGFBP3 were positively correlated in both sexes and with age (P < .01). We found significant differences in the levels of ALS, IGF1 and IGFBP3 and the IGF1/IGFBP3 molar ratio between the sexes and higher levels in pubertal boys (P < .01). We generated normal probability plots for each sex for each of the components of the ternary complex and for the IGF1/IGFBP3 and IGFBP3/ALS molar ratios. In addition, we extracted equations from the models for the calculation of z-scores for age and sex.
ConclusionsThis study may contribute age- and sex-specific reference values for IGF1, IGFBP3 and ALS levels and IGF1/IGFBP3 and IGFBP3/ALS ratios in Spanish children and suggests an association between age, sex, and pubertal stage.
La subunidad ácido-lábil (ALS) tiene un papel importante en los efectos endocrinos de los factores de crecimiento similares a la insulina (IGF) en tejidos diana. Históricamente ha recibido una atención limitada. El objetivo de nuestro estudio fue describir el rango normal de ALS en niños sanos y su relación con otros factores de crecimiento.
Pacientes y métodosSe diseñó un estudio descriptivo transversal. Se recopilaron datos sobre edad, altura, índice de masa corporal, edad gestacional, antropometría al nacer y niveles séricos de ALS, IGF1 e IGFBP3 de niños sanos de 2 a 15 años con estatura estándar. Los niveles de ALS, IGF1 e IGFBP3 se midieron mediante ELISA. Se utilizaron modelos de normalización GAMLSS para la estandarización de variables.
ResultadosSe recogieron muestras de 446 niños. En niños prepúberes, los niveles de ALS, IGF1 e IGFBP3 se correlacionaron de manera positiva en ambos sexos y con la edad (p < 001). Los niveles de ALS, IGF1 e IGFBP3 y la relación molar IGF1/IGFBP3 fueron significativamente diferentes entre ambos sexos y más altos en los niños puberales (p < 001). Se realizaron gráficas de normalidad por género para cada uno de los componentes del complejo ternario y para las relaciones molares IGF1/IGFBP3 e IGFBP3/ALS. Además, se construyeron fórmulas modelo para calcular el Z Score según la edad y el sexo.
ConclusionesEste estudio podría determinar valores de referencia específicos por edad y sexo para IGF1, IGFBP3, ALS, IGF1/IGFBP3 e IGFBP3/ALS en niños españoles y parece establecer la relación entre edad, sexo y estadio puberal.
Growth is a very complex genetic process regulated by multiple extragenic factors. The standard adult height is only reached when all factors perform adequately. The most important endogenous factors involved in growth are hormones, particularly the growth hormone (GH) axis.1
Insulin-like growth factor type 1 (IGF1), stimulated by GH, plays a key role in growth during childhood and puberty. It is chiefly found in a ternary complex with insulin-like growth factor binding protein-3 (IGFBP3) and the acid-labile subunit (ALS).2
The ALS is a glycoprotein of 84−86 kDa synthetized exclusively at the liver from gene IGFALS (OMIM 615961) and stimulated by GH. It was first described and purified from human serum by Baxter3 in 1990.
The ALS is present almost exclusively in serum and circulates in excess compared to the other components of the ternary complex, with 50%–60% of the ALS found in its free form.3 The first case of short stature caused by ALS deficiency was described in 2004 by Domené et al.4 Since then, 42 variants of the IGFALS gene have been reported.5,6
Recent studies that used massive sequencing have shown that complete ALS deficiency accounts for 3%–6% of children with a diagnosis of GH insensitivity.7,8
Among children with idiopathic short stature, complete ALS deficiency would account for this condition in 1%, while 5% have a blood chemistry profile suggestive of partial ALS deficiency.9
Despite the important role of the ALS in the endocrine effects of insulin-like growth factors (IGFs) on target tissues, it has received limited attention in the past compared to other circulating components of the IGF1 system.10
The importance of measuring the levels of ALS in children with short stature has yet to be established.
The characterization of children with short stature and partial ALS deficiency could be clinically relevant, as these patients have exhibited an increase in growth rate after starting treatment with GH.9,11
The aim of our study was to establish the normal range of ALS in health children to identify the factors that affect the concentration of ALS and the association with other growth factors.
Patients and methodsWe conducted a cross-sectional descriptive study. The study was carried out in a referral paediatric endocrinology unit in collaboration with the school of medicine of the same city.
The inclusion criteria were: healthy child aged 2–15 years, height z score greater than –2 body mass index (BMI) z score between –2 and 2, gestational age (GA) of at least 36 weeks, birth weight and birth length z scores between –2 and 2. All anthropometric values were adjusted for sex, age and GA using the tables of the 2010 growth study in Spain as reference.12
We collected data on age, height, weight and BMI at the time of sample collection, in addition to the serum levels of ALS, IGF1 and IGFBP3, GA, birth length and birth weight.
We collected serum samples from healthy children under different circumstances: patients referred to a paediatric endocrinology clinic who did not have disease, healthy children in primary care centres and children who were scheduled for an intervention for mild, non-chronic, non-inflammatory disease (inguinal hernia, phimosis, ingrown nail, unilateral cryptorchidism, prominent ears …). We excluded patients with any form of disease that could affect the results and patients with incomplete health records.
The legal guardians of participants and all children older than 12 years with sufficient maturity signed the informed consent form.
The study was conducted in adherence to the ethical principles of the Declaration of Helsinki and was approved by the ethics committee of the Hospital Universitario de Salamanca.
The total levels of ALS were measured by enzyme-linked immunoassay (ELISA) (ALS E35 Assay, Mediagnost Laboratory, Germany) with rabbit specific antibodies.13,14 The limit of detection was 0.23 mU/mL; the inter-assay variability was less than 8%, and the intra-assay variability less than 6.8%.
The total concentration of IGF1 was measured with a solid-phase, enzyme-labelled chemiluminescent immunometric assay (IMMULITE 1000 IGF-I, Siemens). An acid treatment was used to separate IGF1 from binding proteins, leaving IGF1 uncomplexed and exposed to high titres of specific antibodies. The limit of detection was 14.4 ng/mL. The intra-assay and inter-assay coefficients of variation were less than 4.8% and 5.7%, respectively. There was no significant cross-reactivity.
Durante the study period, there was a change in the method used by the laboratory to measure IGF1, with the adoption of a new standard (WHO NBSC IS 02/254). This modified the total levels of IGF1 obtained with the previous standard (WHO NBSC IS 87/518), which turned out to be lower using the new method. The values of the new method exhibited a very strong correlation and very high agreement with the values obtained with the former method, as demonstrated by a study carried out in our laboratory, in which 95.6% of the results were within optimal limits.15 We converted the IGF1 values obtained with the former method with the following equation:
to be able to compare all values of IGF1 under equal conditions.The total levels of IGFBP3 were measured by chemiluminescence (IDS-iSYS Insulin-like Growth Factor Binding Protein 3, IS-4400). In this assay, following the addition of activation reagents, the resulting light emitted by the acridinium label is directly proportional to the concentration of IGFBP3 in the original sample. The limit of detection was 50 ng/mL. The intra-assay and inter-assay coefficients of variation were less than 2.6% and 7.2%, respectively. There was no cross-reactivity with other IGFBPs.16
Since the molecular weights of IGF1 (7.6 kDa), IGFBP3 (29 kDa) and ALS (85 kDa) are very different, their ratios must be expressed in terms of moles. The IGF1 to IGFBP3 ratio can be obtained after calculating both concentrations in nanomol/L. Given the substantial difference in molecular weight between IGF1 and IGFBP3, the molar ratio expressed as a percentage provides a measure of the relative abundance of IGF1 compared to IGFBP3.16–18 Our calculation of the IGF1/IGFBP3 ratio was based on the method published by Friedrich et al.16
Statistical analysisWe assessed the normality of the data by means of the Shapiro–Wilk and Kolmogorov-Smirnov tests; the distribution was considered normal if the P value was greater than 0.05. We calculated the mean and standard deviation (SD) for normally distributed variables and the median and interquartile range (IQR) for variables that did not follow a normal distribution. We compared groups by means of the Student t test and analysis of variance (ANOVA) if the data followed a normal distribution, and otherwise by means of the Mann–Whitney U and Kruskal–Wallis tests.
We used generalized additive models for location scale and shape (GAMLSS)19,20 to standardize the molar values of ALS, IGF1 and IGFBP3, and the IGF1/IGFB3 and IGFBP3/ALS ratios in the healthy paediatric population. We adjusted the models for age and sex. The statistical analyses were performed with the software SPSS version 2121 and R.22
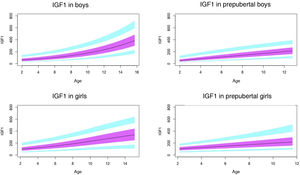
Standard scores or z scores represent the number of SDs that a value is above or below the mean or median of the reference population. In this method, the values of the population follow a normal distribution (Laplace–Gauss).
The equation used to calculate z scores was: z = (difference of observed value and the mean value in the reference population)/[SD in the reference population(ln)].
The reference values for the variables in our study (ALS, IGF1, IGFBP3, IGF1/IGFBP3 and IGFBP3/ALS) do not follow a normal distribution, so it was necessary to make a logarithmic transformation of the data before proceeding to their standardization in order to later calculate the z scores.23
ResultsWe collected samples from 446 children (267 boys and 179 girls) with a median age of 7.6 years.
General descriptionWe collected data for 343 prepubertal children (222 boys and 121 girls) and 103 pubertal children (45 boys and 58 girls).
The median age was 7.1 years (4.73–10.66) in boys and 8.2 years (5.98–10.98) in girls. The mean height z score was 0.06 (SD, 0.99) in boys and 0.03 (SD, 1.06) in girls. We did not find significant differences in the height, weight, BMI or anthropometric values at birth between male and female participants. The characteristics of the sample are summarised in supplemental Table 1 (Appendix A).
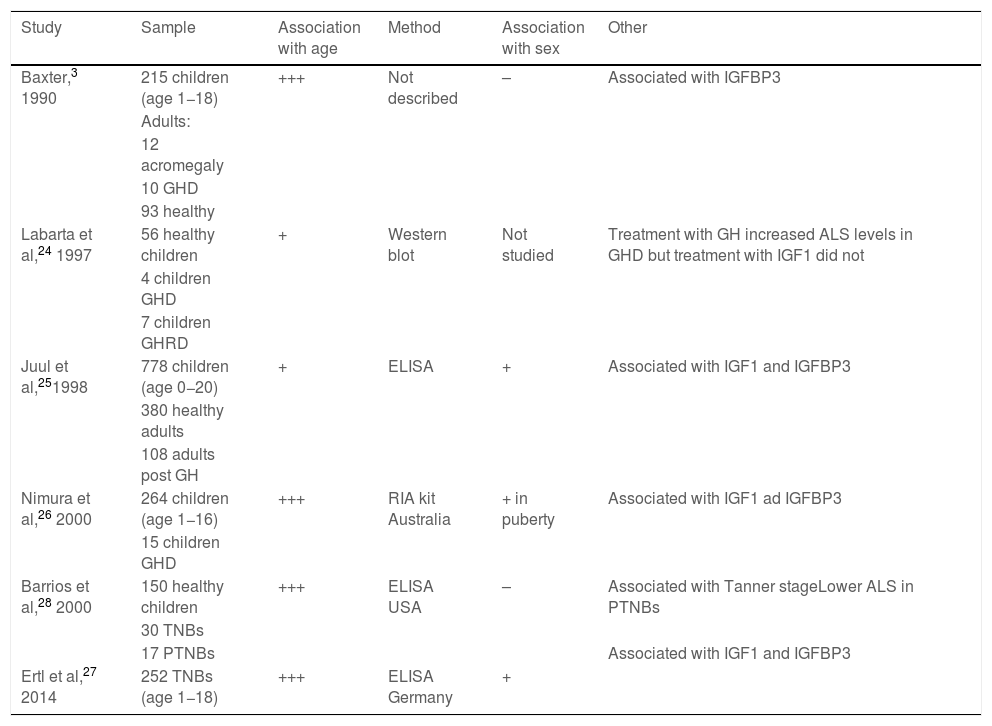
Results of previous studies on the normal value range of acid-labile subunit.
| Study | Sample | Association with age | Method | Association with sex | Other |
|---|---|---|---|---|---|
| Baxter,3 1990 | 215 children (age 1−18) | +++ | Not described | – | Associated with IGFBP3 |
| Adults: | |||||
| 12 acromegaly | |||||
| 10 GHD | |||||
| 93 healthy | |||||
| Labarta et al,24 1997 | 56 healthy children | + | Western blot | Not studied | Treatment with GH increased ALS levels in GHD but treatment with IGF1 did not |
| 4 children GHD | |||||
| 7 children GHRD | |||||
| Juul et al,251998 | 778 children (age 0−20) | + | ELISA | + | Associated with IGF1 and IGFBP3 |
| 380 healthy adults | |||||
| 108 adults post GH | |||||
| Nimura et al,26 2000 | 264 children (age 1−16) | +++ | RIA kit Australia | + in puberty | Associated with IGF1 ad IGFBP3 |
| 15 children GHD | |||||
| Barrios et al,28 2000 | 150 healthy children | +++ | ELISA USA | – | Associated with Tanner stageLower ALS in PTNBs |
| 30 TNBs | |||||
| 17 PTNBs | Associated with IGF1 and IGFBP3 | ||||
| Ertl et al,27 2014 | 252 TNBs (age 1−18) | +++ | ELISA Germany | + |
ALS, acid-labile subunit; GH, growth hormone; GHD, growth hormone deficiency; GHRD, growth hormone receptor deficiency; IGFBP3, insulin-like growth factor binding protein-3; IGF1, insulin-like growth factor type 1; PTNB, preterm newborn; TNB, term newborn.
In prepubertal children, the levels of ALS, IGF1 and IGFBP3 were significantly and positively correlated in both sexes and with age (P < .01). The correlation with age disappeared with puberty, although this could be due to the small sample size, as the study only included 45 pubertal boys and 58 pubertal girls.
The levels of ALS, IGF1 and IGFBP3 were positively correlated to height and the BMI z score (P < .01), but not with the birth length z score. The IGF1/IGFBP3 molar ratios were also positively correlated to age, height and BMI (P < .05).
Comparison of IGF1, IGFBP3 and ALS based on sex and pubertal stage (Appendix A, Supplemental Table 4)The levels of ALS, IGF1 and IGFBP3 and the IGF1/IGFBP3 ratio were significantly different in boys and girls and significantly higher in pubertal children compared to prepubertal children (P < .01).
We did not find significant differences in the IGFBP3/ALS ratios between boys and girls or between pubertal and prepubertal children.
We did not find significant differences in ALS levels based on the Tanner stage (II, III, IV or V) in pubertal children.
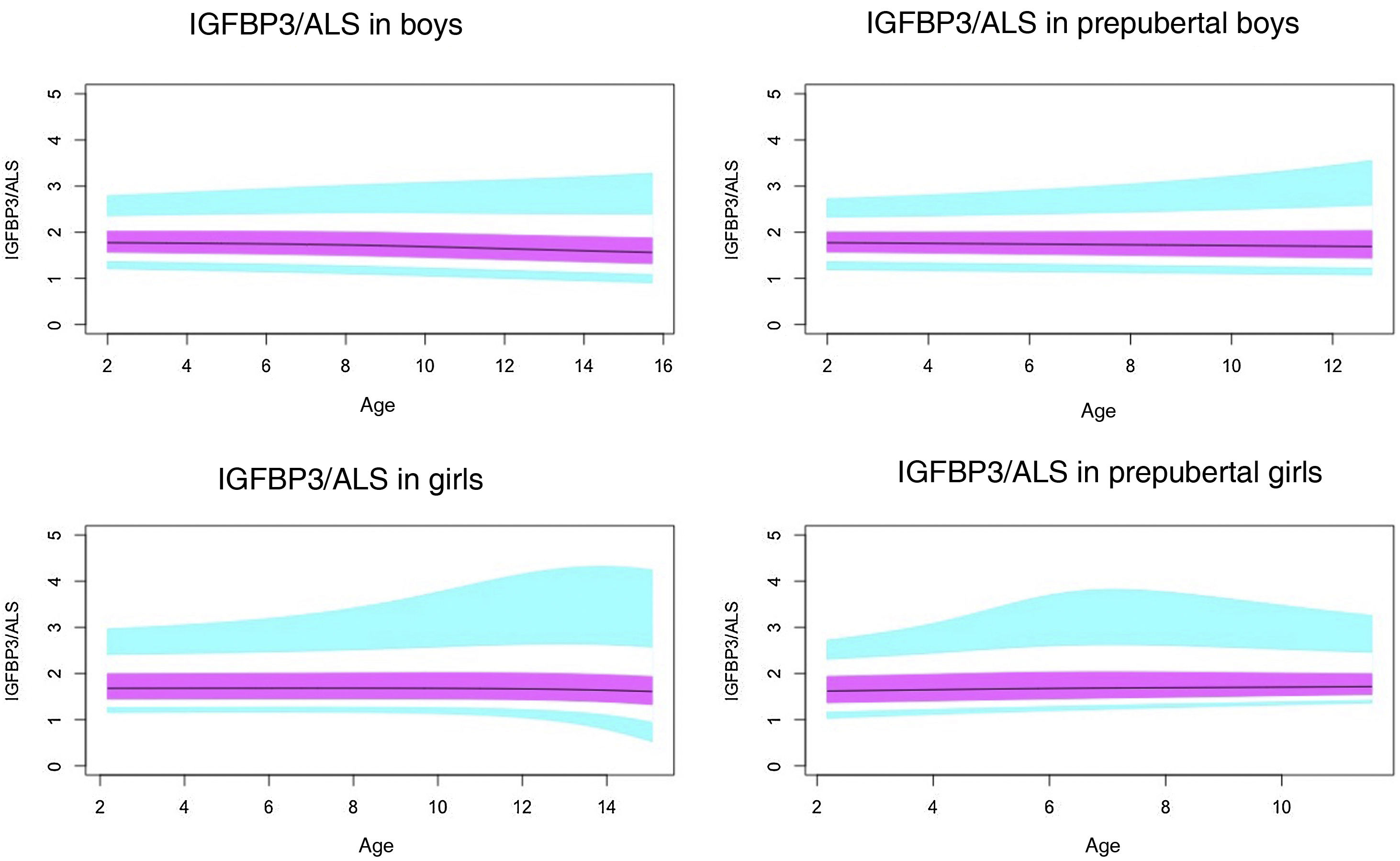
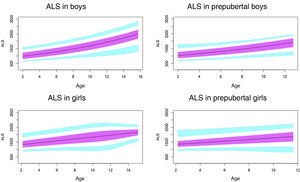
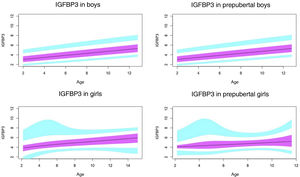
Normal probability plotsWe generated normal probability plots for each sex for the entire sample. We also generated plots for the values of each of the components of the ternary complex and for the IGF1/IGFBP3 and IGFBP3/ALS molar ratios for the entire sample and the prepubertal group (Figs. 1–5).
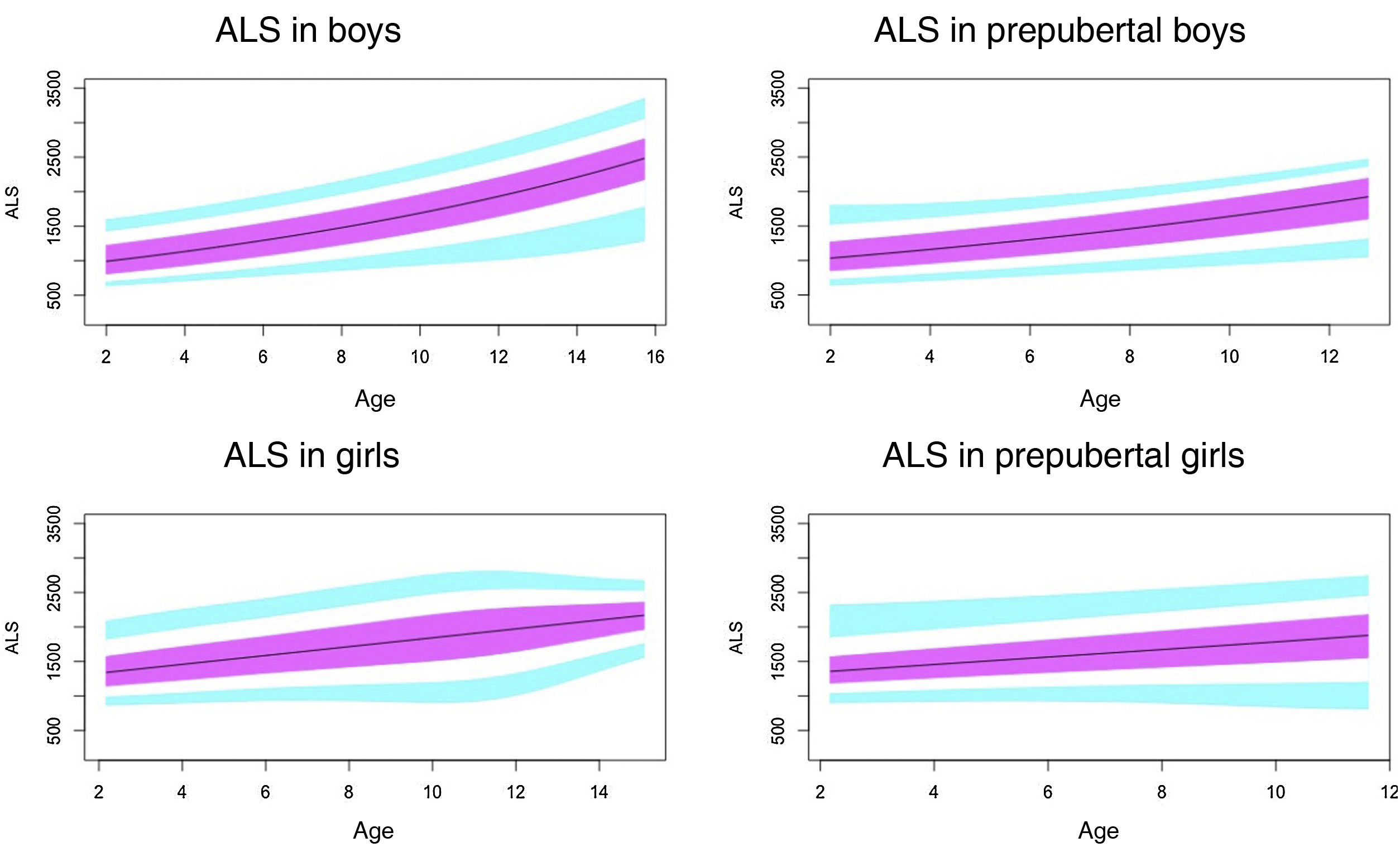
Normal value range for the acid-labile subunit (ALS) in mU/mL.
The blue line corresponds to the values of the 3rd to 10th percentiles and the 90th to 97th percentiles, the purple line to the values between the 25th and 75th percentiles, and the black line to the z score value of 0, or 50th percentile.
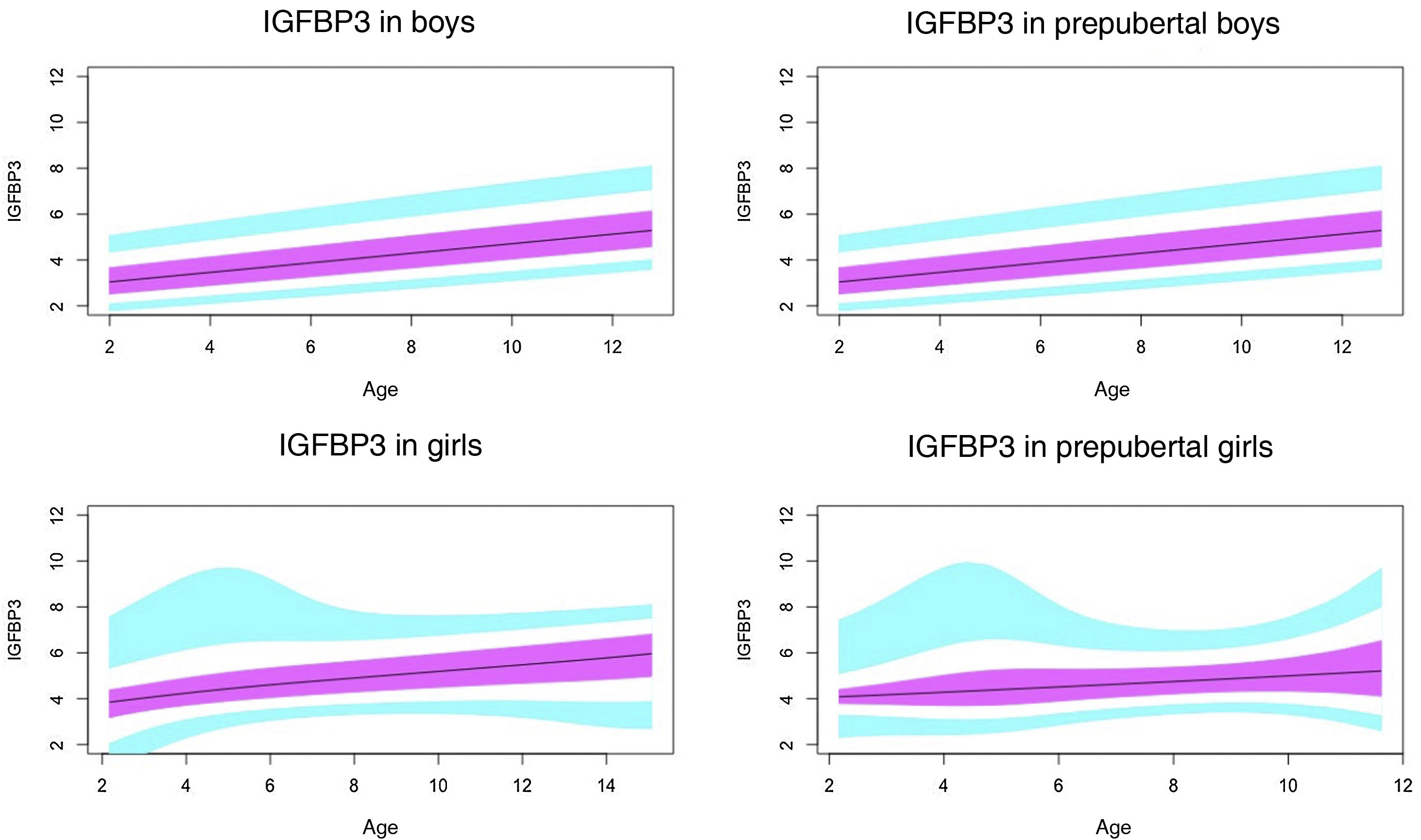
Normal value range for insulin-like growth factor binding protein-3 (IGFBP3) in μg/mL.
The blue line corresponds to the values of the 3rd to 10th percentiles and the 90th to 97th percentiles, the purple line to the values between the 25th and 75th percentiles, and the black line to the z score value of 0, or 50th percentile.
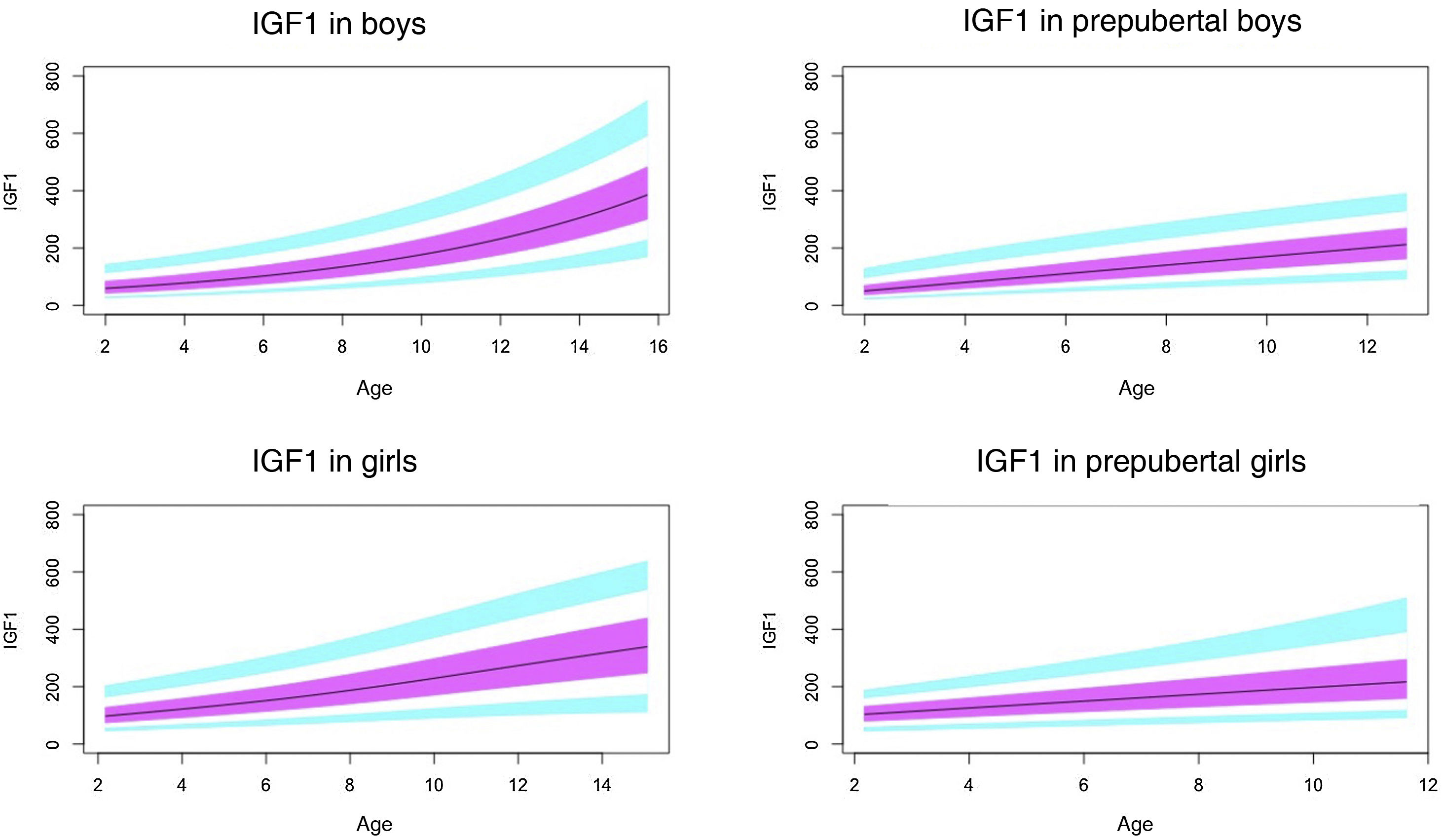
Normal value range for insulin-like growth factor type 1 (IGF1) in ng/mL.
The blue line corresponds to the values of the 3rd to 10th percentiles and the 90th to 97th percentiles, the purple line to the values between the 25th and 75th percentiles, and the black line to the z score value of 0, or 50th percentile.
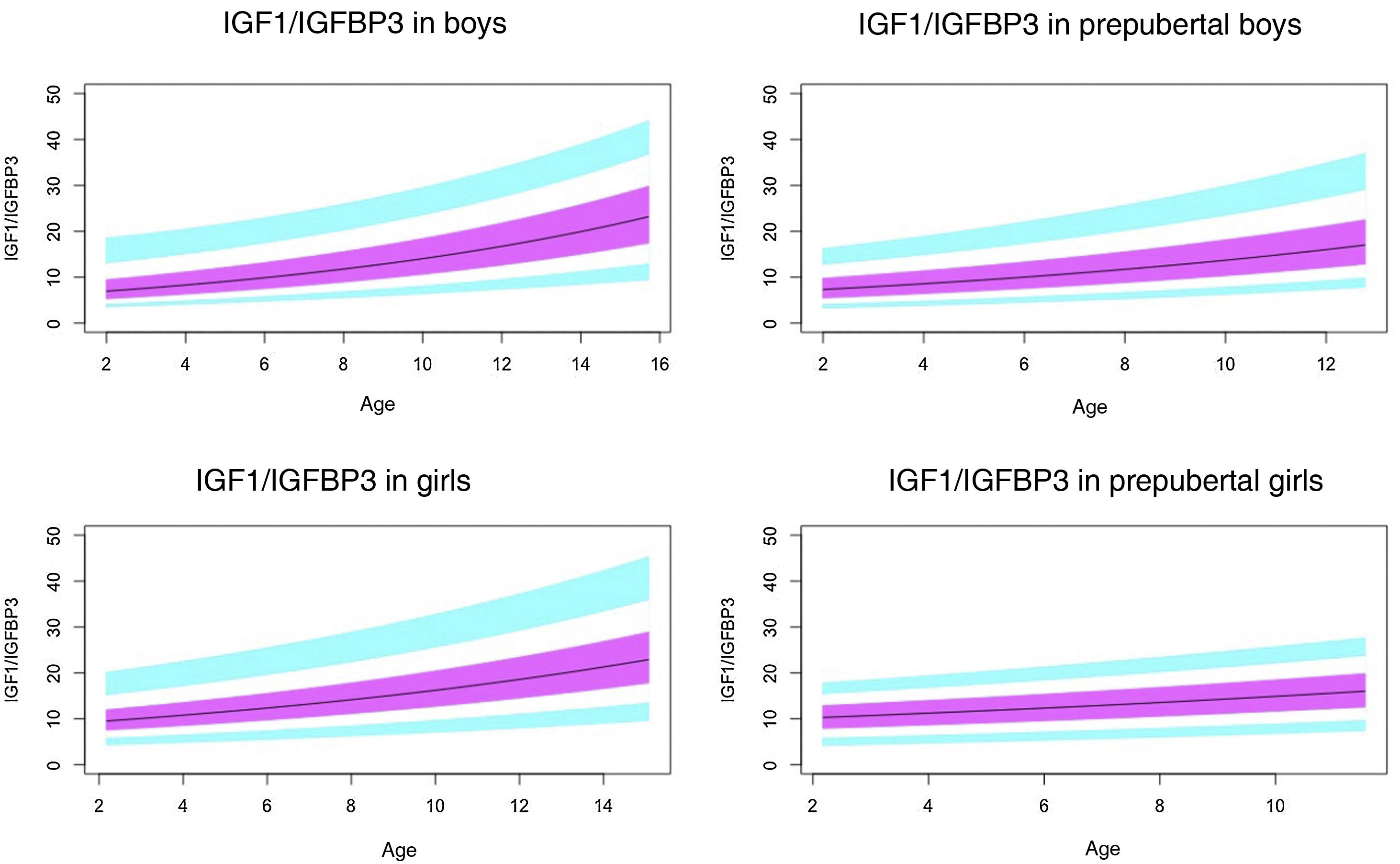
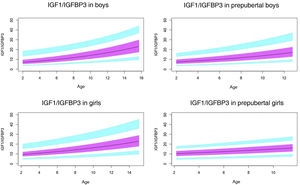
Normal value range for the IGF1/IGFBP3 molar ratio.
The blue line corresponds to the values of the 3rd to 10th percentiles and the 90th to 97th percentiles, the purple line to the values between the 25th and 75th percentiles, and the black line to the z score value of 0, or 50th percentile.
IGFBP3, insulin-like growth factor binding protein-3; IGF1, insulin-like growth factor type 1.
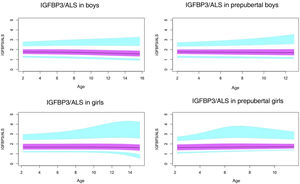
Normal value range for the IGFBP3/ALS molar ratio.
The blue line corresponds to the values of the 3rd to 10th percentiles and the 90th to 97th percentiles, the purple line to the values between the 25th and 75th percentiles, and the black line to the z score value of 0, or 50th percentile.
ALS, acid-labile subunit; IGFBP3, insulin-like growth factor binding protein-3.
After performing the logarithmic transformation of the data to achieve a normal distribution, we were able to calculate the z score corresponding to observed values with the following formula: z = [ln (observed value) – ln (median value in the reference population)]/[SD in the reference population (ln)]
The mean values and SD values for the reference population can be found in Supplementary Tables 5–9 in Appendix A.
DiscussionThis study was conducted to determine the normal range of ALS in the healthy paediatric population through the creation of tables, formulas and normal probability plots. It also established the normal range for the IGF1/IGFBP3 and IGFBP3/ALS molar ratios through tables, formulas and normal probability plots.
We found that the levels de ALS increase progressively with age throughout the development of the child.
The levels of ALS were associated with the concentrations of IGF1 and IGFBP3 and with age, as found in previous studies3,24–28 (Table 1). The reported differences between the sexes have been heterogeneous in the previous literature (Table 1).
Our study found differences in ALS levels between the sexes in children. Girls had higher levels of ALS, especially in the prepubertal period, as was the case in the studies by Ertl et al.27 and Juul et al.,25 and in opposition to other studies that did not find differences between the sexes28 or only found differences in the pubertal period.26
Our results show a rapid increase in ALS levels in the pubertal period, starting from 10 years of age, and a moderate increase in prepubertal children before age 10. This differs from the study of Ertl et al.,27 in which ALS levels increased rapidly from age 12 to 13 years. This could be explained by the presence of prepubertal and pubertal participants in the study.
The method and unit of measure used in the study conducted by Barrios et al.28 differed from ours, but both studies found an increase in the levels of ALS with age and pubertal stage.
In our study, we observed variations in IGF1 and IGFBP3 throughout development. The levels increased with age, and the increase was greater in the pubertal period. We also found differences based on sex, as described by other authors.29–35
Based on the literature, the pubertal peak in IGF1 occurs earlier in girls.38,39 This could be due to the onset of puberty occurring at a later chronological age in boys. We found that IGF1 levels tended to plateau in pubertal girls at around age 14 years. This did not occur in boys, who in our sample could be as old as 15 years, as puberty in males ends later. Ertl et al.27 also observed this plateau in girls aged more than 14 years, while in boys the plateau and subsequent decrease occurred from age 17 years. The study by Alberti et al.31 also found a decreasing trend in girls from age 14 years and later in boys.
In agreement with previous studies,30,31 we found differences in the levels of IGFBP3 based on sex.
In the prepubertal period, up to age 10 years, IGFBP3 levels were higher in girls, which was consistent with previous reports.35–38
In our study, we found an increase in IGFBP3 associated with age throughout the prepubertal period, both in boys and girls, and a plateau, or even a decreasing trend, from age 14–15 years and in Tanner stage III, which was also described by Juul et al.30
Previous studies, like the one conducted by Ertl et al.,27 have also found a plateau in the levels of IGFBP3 from age 14 years in boys and age 12 in girls, but they did not analyse differences based on pubertal stage.
The IGF1/IGFBP3 ratio increased with age. The increase was quicker in the pubertal period. The ratio is significantly higher in pubertal children compared to prepubertal children, which suggests that in the pubertal period, the increase in IGF1 is greater than the increase in IGFBP3. This is consistent with the greater growth rate observed in previous studies.30,31,35,39
The IGFBP3/ALS molar ratio remained all but constant throughout childhood, without significant differences between pubertal and prepubertal children. The ALS only binds IGFBP3 when the latter is in a binary complex with IGF1, and the affinity of ALS for the binary complex is much lower compared to the affinity of GFBP3 for IGF1. The IGFBP3/ALS molar could be an important parameter to assess in children with partial ALS deficiency, as it could reveal a decreased amount of ALS.
Previous studies on the association between growth factors and anthropometric measurements have yielded heterogeneous results.30,32–35 Our study found a positive correlation between the levels of IGF1, IGFBP3, ALS and the IGF1/IGFBP3 ratio with the height and BMI z scores, but not with birth length. The latter is an exception in that during prenatal development, insulin-like growth factors do not require GH for their expression or action. There is robust evidence that GH does not play an important role in prenatal growth. Thus, patients with severe GH deficiency (even those with GH deficiency type IA, which entails the complete absence of GH) and patients with Laron syndrome caused by genetic changes that disable the GH receptor, have birth weights and lengths within the normal range. On the other hand, patients with complete IGF1 or IGF2 deficiency exhibit a characteristic picture of intrauterine growth restriction, which evinces the important role played by both growth factors on intrauterine growth. We ought to highlight that during this stage of development, normal expression of growth factors IGF1 and IGF2 does not require the action of GH.
LimitationsThe study included a substantial number of healthy children, but it has limitations.
First, the number of pubertal participants in the study was not sufficient to produce reliable normal probability plots, and the age range of participants was restricted to 2–15 years, so we were unable to clearly establish the changes that take place during puberty, which would have required the inclusion of participants up to age 18 years.
Secondly, the discontinuation of the assay initially used for the determination of serum concentrations of IGF1 in the study (WHO NBSC IS 87/518) required the conversion of each serum IGF1 value obtained with the previous standard to an equivalent value for the assay currently in use (WHO NBSC IS 02/254).15
ConclusionThe combined measurement of IGF1, IGFBP3 and ALS could be useful in the diagnosis and follow-up of children with growth disorders.
In this study, we obtained reference values for age and sex for the levels of IGF1, IGFBP3 and ALS and the IGF1/IGFBP3 and IGFBP3/ALS ratios in Spanish children. We established the association with age, sex and pubertal stage and developed a formula to calculate z scores for age and sex.
These standardized data could facilitate the management of paediatric patients and the monitoring of healthy children.
The generation of normal probability plots for sex, age and pubertal stage of molar ratios linking the different components of the ternary complex could be useful in the evaluation and follow-up of patients with growth disorders.
Larger studies are required to adjust the normal range, especially in pubertal children.
FundingThe study did not receive any specific grants from funding agencies in the public, private or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to disclose that could be construed as compromising impartiality in the reported research.