S100β protein has been proposed as a potential biomarker for both chronic and acute neurological disorders. Reference values of this protein are well defined in adults but not in children, in whom serum levels appear to vary with age. Reference values for serum S100β in children from 0 to 14 years are presented.
Materials and methodsA prospective study was conducted on 257 healthy children, who were divided into three age groups (under 12 months, 12–24 months and over 24 months).
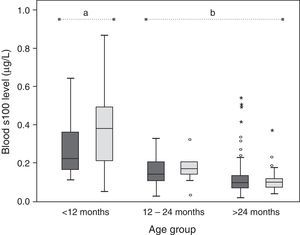
ResultsThe study included 179 boys and 78 girls, with a mean age of 5.5 (3.75) years. The mean serum concentration of protein S100β was 0.156 (0.140–0.172)μg/L. In children under 12 months, serum S100β concentration was 0.350 (0.280–0.421)μg/L; 0.165 (0.139–0.190)μg/L in the group between 12 and 24 months and 0.121 (0.109–0.133)μg/L in children older than 24 months. An inverse relationship was observed between age and serum S100β, which declines as age increases. No differences were observed between sexes.
ConclusionsThe concentration of S100β remains stable after two years of age, being possible to establish a baseline of S100β for over two years. During the first two years of life, S100β serum concentration is higher, the lower the age of the child. No differences in serum S100β levels between sexes are observed.
La proteína S100β se ha propuesto como posible biomarcador en patología neurológica, tanto crónica como aguda. Los valores normales de esta proteína están bien definidos en adultos, no así en niños, en los que los valores séricos parecen variar con la edad. Nuestro objetivo es describir valores de referencia de S100β sérica en niños de 0 a 14 años.
Material y métodosEstudio prospectivo en 257 niños sanos. Se establecieron 3 grupos por edad (menores de 12 meses, de 12 a 24 meses y mayores de 24 meses).
ResultadosSe incluyó a 179 niños y 78 niñas. La edad media ± DE fue de 5,5 ± 3,75 años. La concentración sérica media de la proteína S100β en todo el grupo fue 0,156 (0,140-0,172) μg/l. En los menores de 12 meses, la concentración sérica de S100β fue de 0,350 (0,280-0,421) μg/l; 0,165 (0,139-0,190) μg/l en el grupo entre 12 y 24 meses y 0,121 (0,109-0,133) μg/l en el grupo de niños mayores de 24 meses. Se observó una relación inversa entre la edad y la concentración sérica de S100β, que desciende conforme se incrementa la edad. No se observaron diferencias en cuanto al sexo.
ConclusionesLa concentración de S100β permanece estable a partir de los 2 años de edad, siendo posible establecer unos valores de referencia de S100β para mayores de 2 años. En los 2 primeros años de vida, la concentración de S100β sérica es más elevada cuanto menor es la edad del niño. No se observan diferencias en el valor de S100β sérica entre ambos sexos.
The use of biomarkers has proven helpful in the management of different diseases. Data for the paediatric population are usually scarce due to the restrictions placed on research studies. Thus, the concentration patterns of different markers, initially studied in adults, are usually extrapolated to children, as has been the case of protein S100β. This protein, which is predominantly found in astroglial and Schwann cells,1 is secreted by astrocytes as a cytokine with neurotrophic and gliotrophic effects.2–4 Under normal conditions, it is detected in minimal concentrations in peripheral blood. Its mean half-life ranges between 25min and 2h.5–8 This protein may be of interest in cases of acute neurologic pathology, such as brain injury or neonatal hypoxic-ischaemic encephalopathy. Diagnostic strategies based on biomarkers have been proposed as an alternative to computed tomography in the management of brain injury.9,10
The serum concentrations of S100β reported in healthy adults range between 0.02 and 0.05μg/dL.11–14 These values cannot be extrapolated to children, as serum levels of S100β seem to change with age,1,3,15 with higher levels found in the first three years of life.16 Furthermore, serum concentrations of S100β vary depending on the method used for its determination.17 All these are barriers to establishing reference values for children, which nevertheless are essential in order to be able to use this protein in different diagnostic and prognostic scenarios.
Thus, the aim of our study was to determine the serum concentrations of S100β in healthy children from birth to 14 years of age in our setting (Hospital Universitario Central de Asturias).
Patients and methodsWe conducted a descriptive prospective study at the Hospital Universitario Central de Asturias (HUCA) between June 2008 and May 2010, following approval by the Regional Clinical Research Ethics Committee. We obtained the signed informed consent of the parents or guardians of all children included in the study.
We collected blood samples from healthy children aged 0–14 years as part of the preoperative evaluation for minor surgeries. In addition to these samples, we included blood samples from children seen at the paediatric emergency department that were collected for a variety of processes. We excluded samples obtained from children with diseases that could lead to elevated S100β serum concentrations, such as acute or chronic diseases of the central nervous system (CNS), bone fractures, Down syndrome, kidney failure, or developmental delay, and samples from children that had experienced a febrile seizure in the week preceding the blood being drawn.
We divided the sample into three age groups: age less than 12 months, age between 12 and 24 months, and age more than 24 months.
All samples were collected in bottles without an anticoagulant and submitted to the department of clinical biochemistry (Medicine Laboratory) of the HUCA. They were centrifuged at 3000rpm for 10min (Labofuge 400R; Heraeus, Boadilla, Spain). The serum was then collected and stored at −80°C until testing. The determination of S100β protein in serum samples was performed by electrochemiluminescence using a Cobas e-601 analyser (Roche Diagnostics, Mannheim, Germany), which performs a sandwich protocol with two incubation periods and a total testing time of 18min. Each determination was performed twice, following manufacturer's recommendations. The lower detection limit for the method was 0.005μg/L, and the variability coefficient less than 2%.
Data analysisWe performed the statistical analysis with the software SPSS version 18 (SPSS Inc, Chicago, USA). We have expressed the data as means±standard deviations (SDs), medians, and 95% confidence intervals (CIs). After checking the normality of the distributions by interpretation of Q–Q plots, we analysed the differences between groups with Student's t test (two groups) and multifactorial ANOVA with the Tukey HSD post hoc test (more than two groups). We set the level of statistical significance at P<.05.
ResultsWe included 257 patients in the study, of whom 179 were male (69.7%) and 78 female (30.4%). More than half of the blood samples (67.3%) were obtained from preoperative evaluations.
The mean age and standard deviation of the boys were 5.5±3.75 years, with a median of 4.76 years. In girls, the mean age was 4.61±4.19 years and the median 3.40 years. We did not find statistically significant differences in age between the sexes (Student's t, P=.096), and 26.8% of the children were aged less than 2 years.
The mean serum concentration of the S100β protein in the overall sample was 0.156 (0.140–0.172)μg/L. The means in each age group were 0.350 (0.280–0.421)μg/L in children aged less than 12 months, 0.165 (0.139–0.190)μg/L in children aged 12–24 months, and 0.121 (0.109–0.133)μg/L in children aged more than 24 months.
When we considered the sex of the patients, the concentration was significantly higher in girls (0.191 [0.153–0.230]μg/L) than in boys (0.141 [0.126–1.156]μg/L) (Student's t, P=.018). The analysis by age group did not show significant differences between the sexes (P=.137, P=.690 and P=.129, respectively).
The multifactorial ANOVA showed that the mean serum concentrations of S100β differed significantly between age groups (P<.001), while a significant difference in mean concentrations was not found between the sexes (P=.141). There was a significant interaction effect between both factors (P=.017), which indicates that the observed protein concentration differences between age groups are not the same in both sexes. The serum levels of S100β were higher in females than in males (although these differences were not statistically significant), especially in the youngest age group. As for the differences observed between age groups, the post hoc analysis showed that the protein concentration was significantly higher in the group of individuals aged less than 12 months compared to the other two groups (Fig. 1).
We calculated percentiles and 95% CIs for the three age groups (Table 1). We were unable to calculate the 95% CI for every percentile for the group aged 1–2 years due to the small sample size.
Serum S100β concentrations in the three age groups considered in the study, expressed as mean and 95% confidence interval (CI).
| Percentile | S100β concentration in μg/L (95% CI) | ||
|---|---|---|---|
| <12 months (n=33) | 12–24 months (n=36) | >24 months (n=188) | |
| 2.5 | 0.076 | 0.030 | 0.043 (0.027–0.048) |
| 5 | 0.119 | 0.043 | 0.048 (0.041–0.051) |
| 10 | 0.145 | 0.066 (0.025–0.111) | 0.053 (0.049–0.060) |
| 25 | 0.187 (0.148–0.244) | 0.113 (0.087–0.138) | 0.071 (0.064–0.078) |
| 50 | 0.296 (0.209–0.428) | 0.168 (0.127–0.193) | 0.100 (0.093–0.107) |
| 75 | 0.483 (0.383–0.630) | 0.209 (0.175–0.244) | 0.135 (0.125–0.148) |
| 90 | 0.645 | 0.276 | 0.206 (0.178–0.236) |
| 95 | 0.734 | 0.328 | 0.246 (0.228–0.456) |
| 97.5 | 0.830 | 0.331 | 0.410 (0.249–0.520) |
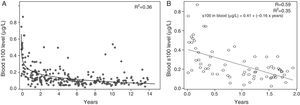
The regression analysis showed an inverse correlation between age and serum concentrations of the protein, which decreased as aged increased, with the decrease being most pronounced in the early years of life. The separate analysis of protein concentrations in patients aged 2 years and less showed a linear relationship between concentration and age, and the regression model obtained was statistically significant (ANOVA, P<.001) (Fig. 2).
(A) Regression analysis: logarithmic curve fit for the entire dataset. The figure shows the goodness of fit of the model (R2). (B) Linear regression model for the data obtained from patients aged up to 2 years. The figure shows the goodness of fit (R2 and R) and the linear regression equation.
In recent years, there has been a growing interest in the use of biomarkers in the management of different pathologies and biological processes—which requires their determination to be easy, quick and economical—and in learning the normal concentration patterns of these proteins in the populations of interest and the concentration patterns in the pathologies in which they are meant to be applied. When it comes to CNS pathologies, biomarkers with an adequate sensitivity and specificity are not yet available. In the paediatric field, protein S100β could be a potentially useful biomarker in different biological fluids, such as peripheral blood, cord blood, urine, cerebrospinal fluid, etc.
There are often ethical and legal obstacles to performing clinical research in children, which limits the number of patients included in studies. This frequently leads to the extrapolation of values found in adults to paediatric patients. However, the influence of age, which is associated with different degrees of organ and system development and maturation, periods of rapid growth, and differences in hepatic and renal metabolism, may preclude the possibility of making such extrapolations.
To our knowledge, our study is the first conducted in Spain to analyse S100β concentrations in healthy paediatric patients, which is why we had a keen interest in obtaining data on the concentration patterns of this protein in our setting using the method available to us (electrochemiluminescence).
Most of the samples we used were obtained from preoperative evaluations for minor surgical interventions, to which we added samples from children with other conditions (the largest number corresponded to children with fever without source) and observed that this circumstance did not influence serum S100β levels or the presence of bilirubin in blood, which was consistent with what Kleindienst et al. had reported in adults.18
The first studies in paediatric patients did not address the potential effects of age, but observed that concentrations were higher than in adults. The study by Gazzolo et al. was the first to provide data on paediatric patients broken down by age group.2 The highest serum levels of S100β have been reported in healthy infants, decreasing in later ages.3,19 Higher concentrations of S100β in newborns with lower gestational ages have been found in other biological fluids.20,21 The variation in the serum concentration of this protein between the early months of life and later ages may be due to differences in the blood–brain barrier and brain circulation, as well as to the function of the protein, with evidence of a gradual decrease in the serum concentration of S100β. This reduction could reflect a reduced release of this trophic factor at more advanced stages of brain maturation. Other evidence has described a possible increase in serum S100β from age 7 years on, which may be related to height growth and nerve elongation,2,15 a fact that we did not observe in our sample. This hypothesis is supported by the presence of S100β in peripheral nerves and by its stimulating action on neurite outgrowth.2
Previous studies on serum S100β concentrations in children have provided few data for the first years of life, and it is common for the youngest age group to be the least represented3 or for the age distribution of the children to not be reported,1 with the exception of the study conducted by Bouvier.16 This may be related to the difficulty of obtaining samples in this age group. Thus, we considered that including 69 children in this age range in the study would provide highly relevant data for elucidating concentration patterns of S100β in this age group.
We also observed an inverse correlation between serum S100β concentrations and age, consistent with previous evidence,2 with concentrations being significantly higher in the first 2 years of life and essentially starting to decrease from age 1 year until reaching a stable mean value for all subsequent age groups.
Different age intervals have been suggested for analysis, and reference values for serum S100β concentrations in children aged more and less than 3 years proposed in recent years, with mean S100β values similar to the mean for our sample.9,16,22 The differences observed between the various studies that use electrochemiluminescence methods are probably due to differences in the age distributions of their samples. Our findings suggest that serum S100β concentrations are stable from age 2 years with levels similar to those described in adults,9,22,23 which makes it possible to establish reference values for children aged more than 2 years, in whom the mean serum concentration of S100β is 0.120μg/L (0.083) with a 50th percentile of 0.100μg/L. In a study of a sample of 186 children, Bouvier proposed calculating reference values for different age subgroups in children aged less than 3 years (0–3 months, 4–9 months, 10–24 months, 25–36 months) and found no significant differences between the groups.16 While it is possible that such differences in the serum concentrations of S100β do exist in the early months of life, we consider that these divisions are not too practical. In our sample, we identified two age subgroups in children younger than 2 years with significantly different values (P<.001). Thus, we propose reference values for children aged less than 1 year, children aged 1–2 years, and children aged more than 2 years. Since one of the potential clinical applications of the determination of protein S100β levels would be its use in the management of children with traumatic brain injury, the age groups we propose would fit those proposed for the management of this pathology.24,25
Gazzolo et al. reported higher concentrations of S100β in females,21 which they attributed to sex differences in the pattern of cerebral maturation, both in the intrauterine period and in adulthood,2 while the potential interaction of sex hormones with S100β cannot be ruled out. However, subsequent studies have not observed these differences in healthy children.3,26 When we compared S100β levels in males and females in our sample for each age group (because variations in the sex distribution of different age groups could be a source of bias), we found no significant differences in the serum concentrations of this protein. However, the serum S100β levels seemed higher in females in the youngest age group.
We think it is worth noting that the reference values for S100β protein serum concentrations described in this study are the first proposed for the paediatric population of Spain.
Limitations of the study- 1.
The study was conducted in a single centre, so the data may not be representative of other paediatric populations.
- 2.
The study included few children aged less than 2 years.
When we analysed serum S100β values in a sample of healthy children, we observed the following:
- 1.
The concentration of S100β remains stable after age 2 years, so that reference values for S100β can be established for individuals aged more than 2 years.
- 2.
In the first two years of life, the serum concentration of S100β is higher, the younger the child.
- 3.
There were no differences in the serum levels of S100β between the sexes.
XI Grants for Clinical Research and Epidemiology in Paediatrics of the Fundación Ernesto Sánchez-Villares (2008). Funds received: 2500 euro.
Conflicts of interestThe authors have no conflicts of interest to declare
We want to thank the Fundación Ernesto Sánchez Villares for awarding us a grant for the amount of 2500 euro in the XI Convocatoria de Ayudas a la Investigación Clínica y Epidemiológica en Pediatría (2008) for the project titled “Utilidad de la proteína S-100 beta en el estudio de los traumatismos craneoencefálicos leves en una población pediátrica” [Usefulness of S100β protein in the study of mild traumatic brain injury in a paediatric population].
We also thank Roche Diagnostics for their disinterested collaboration in this project.
Please cite this article as: Arroyo Hernández M, Rodríguez Suárez J, Álvarez Menéndez F. Valores de referencia de la proteína S100β en población pediátrica. An Pediatr (Barc). 2016;84:254–259.