Newborns with perinatal indicators of a potential hypoxic-ischaemic event require an integrated care in order to control the aggravating factors of brain damage, and the early identification of candidates for hypothermia treatment.
Patients and methodsThe application of a prospective, populational programme that organises and systematises medical care during the first 6h of life to all newborns over 35 weeks gestational age born with indicators of a perinatal hypoxic-ischaemic insult. The programme includes 12 hospitals (91217m2); two level I centres, five level II centres, and five level III hospitals. The programme establishes four protocols: (a) detection of the newborn with a potential hypoxic-ischaemic insult, (b) surveillance of the neurological repercussions and other organ involvement, (c) control and treatment of complications, and (d) procedures and monitoring during transport.
ResultsFrom June 2011 to June 2013, 213 of 32325 newborns above 35 weeks gestational age met the criteria of a potential hypoxic-ischaemic insult (7.4/1000), with 92% of them being cared for following the programme specifications. Moderate–severe hypoxic-ischaemic encephalopathy was diagnosed in 33 cases (1/1000), and 31 out of the 33 received treatment with hypothermia (94%).
ConclusionsThe programme for the Integrated Care of Newborns with Perinatal Hypoxic-Ischaemic Insult has led to providing a comprehensive care to the newborns with a suspected perinatal hypoxic-ischaemic insult. Aggravators of brain damage have been controlled, and cases of moderate–severe hypoxic-ischaemic encephalopathy have been detected, allowing the start of hypothermia treatment within the first 6h of life. Populational programmes are fundamental to reducing the mortality and morbidity of hypoxic-ischaemic encephalopathy.
El recién nacido con indicadores de potencial evento hipóxico-isquémico perinatal precisa de una atención integral que detecte precozmente si necesita tratamiento con hipotermia y el control de los factores agravantes del daño cerebral en las primeras 6 h de vida.
Pacientes y métodosAplicación de un programa prospectivo de ámbito poblacional que ordena y sistematiza la atención durante las primeras 6 h de vida en los ≥ 35 semanas nacidos con indicadores de agresión hipóxico-isquémica perinatal. El programa involucra 12 hospitales (91.217 m2), 7 de nivel asistencial i-ii y 5 de nivel iii. Se establecen 4 protocolos: a) detección del recién nacido con potencial agresión hipóxico-isquémica; b) vigilancia de la repercusión neurológica y en otros órganos; c) control y tratamiento de complicaciones, y d) vigilancia y acciones durante el transporte.
ResultadosEntre junio del 2011 y junio del 2013, de 32.325 recién nacidos ≥ 35 semanas, 213 cumplieron criterios de potencial agresión hipóxico-isquémica perinatal (7,4 por 1.000). El 92% siguió la monitorización establecida en el programa; 33 recién nacidos tuvieron encefalopatía hipóxico-isquémica moderada-grave (1 por 1.000) y 31/33 (94%) recibieron tratamiento con hipotermia.
ConclusionesEl programa Atención integral al Recién nacido con Agresión Hipóxico-Isquémica Perinatal ha permitido ofrecer atención integral al recién nacido con indicadores de agresión hipóxico-isquémica perinatal. Se han controlado factores comórbidos agravantes de la lesión cerebral y se han detectado aquellos con encefalopatía hipóxico-isquémica moderada-grave, permitiendo iniciar la hipotermia dentro de las primeras 6 h de vida. Programas de ámbito poblacional son cruciales para disminuir la morbimortalidad asociada a la encefalopatía hipóxico-isquémica.
The occurrence of a hypoxic-ischaemic event (perinatal asphyxia) is indicated by the presence of alterations in foetal heart rate or pH, or by the history of a sentinel episode.1 When this event is potentially large enough to cause tissue damage, the newborn (NB) shows neurological dysfunction (hypoxic-ischaemic encephalopathy) and/or multiple organ dysfunction/damage (hypoxic-ischaemic disease).2 Hypoxic-ischaemic encephalopathy (HIE) is the leading cause of death, severe neurological morbidity and convulsions in full-term NBs in the world, and is responsible for approximately 20% of cases of cerebral palsy in children.3
Therapeutic hypothermia (targeted temperature management) is currently the specific treatment for reducing the morbidity and mortality associated with HIE. Maximum therapeutic effectiveness is obtained when it is initiated as early as possible, within the first 6h of life. This narrow time frame means that rapid and well-organised intervention needs to be implemented within a few precious hours. This intervention protocol must establish precisely which procedures are to be performed at each stage of care: from the delivery room to intensive care, stabilisation, precise detection of the severity of HIE, checking for comorbid factors that could aggravate brain damage, and occasionally urgent transfer of the patient to referral centres that offer these NBs integrated care including hypothermia.4,5
In order to establish an organised intervention protocol aimed at early detection of NBs with HIE who need treatment with hypothermia and at correcting and avoiding factors that aggravate brain damage in the first 6h of life, a population-based programme, Integrated Care of Newborns with Perinatal Hypoxic-Ischaemic Insult (Atención integral al Recién nacido con Agresión Hipóxico-Isquémica Perinatal [ARAHIP]) has been developed, involving 12 hospitals in the regions of Castilla y León and La Rioja. We here present the programme and report on the experience of the first 2 years of its operation (June 2011–June 2013).
MethodologySteps followed in formulating the programme- 1.
Development of the draft text and preparation of the case report form.
- 2.
Analysis of the real possibilities of applying the programme for each centre and appointment of coordinators.
- 3.
Visit to the centres; presentation of the programme and delivery of the material (programme, summary posters/protocols, training video on neurological examination, case report form).
- 4.
Follow-up of the development of the programme once initiated, difficulties, meeting of coordinators.
Hospitals participating in the ARAHIP programme: (a) Hospital Universitario, Burgos (coordinating centre); (b) Hospital Universitario Río Hortega, Valladolid; (c) Hospital Universitario, Salamanca; (d) Hospital San Pedro, Logroño; (e) Hospital Nuestra Señora de Sonsoles, Ávila; (f) Hospital Universitario, León; (g) Hospital General, Segovia; (h) Hospital Santa Bárbara, Soria; (i) Hospital General, Zamora; (j) Hospital El Bierzo, Ponferrada; (k) Hospital Santiago Apóstol, Miranda de Ebro, and (l) Hospital Santos Reyes, Aranda de Duero.
The number of live NBs greater than 35 weeks gestation cared for in these hospitals put together is approximately 16000 per year. In all of them the NBs are attended at birth by a midwife, with the support of the paediatrician in the event of any associated abnormality. As regards the health care level of the hospitals,6 two of them are level I, five are level II and five are level III. In all, a total of 168 paediatricians (66 of them residents), participating in attending deliveries and in neonatal care, were involved in the programme.
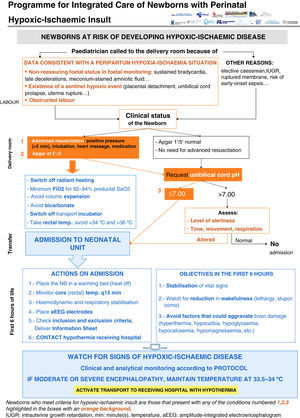
Clinical pathway developed in the programme (Figs. 1–4)Every NB greater than 35 weeks gestation and greater than 1800g at risk of having suffered a perinatal hypoxic-ischaemic insult is included in the programme (Fig. 1). This was defined as meeting at least one of the following criteria: (a) umbilical cord pH of 7.00 or less; (b) Apgar score at 5min of 5 or less, and (c) need for resuscitation with intubation and/or heart massage or need for intermittent positive pressure at 5min. Other supporting but not mandatory criteria for including NBs in the programme were: (a) non-reassuring foetal status (sustained bradycardia, late decelerations or meconium-stained amniotic fluid); (b) existence of a sentinel hypoxic event (placental detachment, umbilical cord prolapse, uterine rupture, foetal exsanguination in the mother), and (c) obstructed labour.
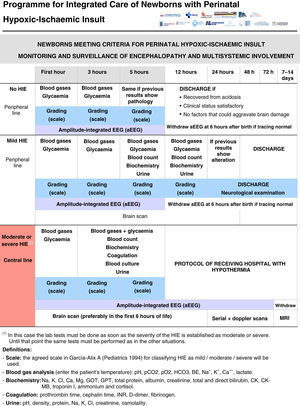
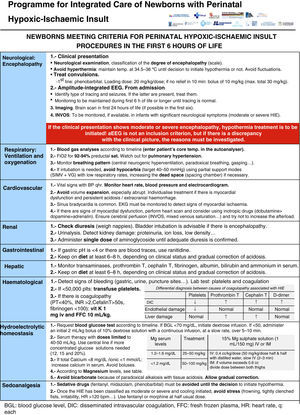
All the NBs that meet the inclusion criteria are enrolled (Fig. 2). The object of enrolment is two-fold: (1) early detection of the presence of moderate or severe HIE and (2) control of factors that could aggravate brain injury or its complications (Fig. 3). To achieve the first objective, systematic neurological examinations are performed at 1, 3 and 5h after birth, and the severity of HIE is established according to the scale proposed by García-Alix et al.7 Hospitals equipped with amplitude-integrated electroencephalography initiate electrocortical monitoring immediately after enrolment and this is maintained until at least 6h after birth or until the tracing normalises (normal voltage, presence of sleep-wake cycling and absence of seizures) If the NB shows moderate/severe HIE in any of the examinations, the receiving referral hospital is contacted for transfer and hypothermia treatment. The area covered by the programme has no established specialised neonatal transport service, so specific instructions have been laid down for managing these children during transfer (Fig. 4).
For the second objective, control of comorbid factors (temperature, hypoglycaemia, hypocarbia, hypomagnesaemia, etc.) with the potential to aggravate brain damage is done by clinical and analytical monitoring (Fig. 2). The management and specific treatment of these comorbid factors are standardised (Fig. 3).
Informed consent is requested from parents in the programme so as to be able to analyse the clinical information derived from it, and it has been approved by the Research Ethics Committee of the coordinating hospital.
ResultsThe ARAHIP population-based programme includes an extensive group of health care facilities with a total area of 91217m2. During the 2 years the programme lasted there were 32325 deliveries of NBs greater than 35 weeks gestation and greater than 1800g.
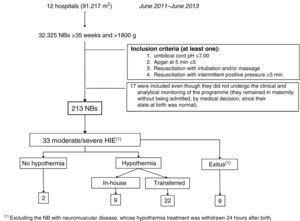
Fig. 5 gives details of the NBs included in the ARAHIP programme and Table 1 shows their main characteristics. Nine of the 12 centres routinely performed cord blood gas analysis for all births. In the remaining three (7636 NBs), blood gas analysis was carried out at the discretion of the physician attending the delivery, in most cases because of perinatal respiratory depression. Taking the 12 participating hospitals as a whole, 213 live NBs met the criteria for perinatal hypoxic-ischaemic insult. This represents a total incidence of 6.6 per thousand live births (95% CI, 5.7–7.5), which, if we exclude the three hospitals without routine pH measurement, increases slightly to 7.4 per 1000 live births (183/24689; 95% CI, 6.3–8.6). Of the 213 BNs with criteria, 64 showed HIE in the first 6h of life (2 per 1000 live births; 95% CI, 1.5–2.5): 31 mild, 23 moderate and 10 severe. The incidence of moderate/severe HIE was one per 1000 (95% CI, 0.7–1.4).
General characteristics of the 213 newborns included in the programme.
| Characteristic | Value (n=213) |
|---|---|
| Gestational age, mean±SD (weeks) | 39.1±1.7 |
| Birth weight, mean±SD (g) | 3133±537 |
| Male, n (%) | 114/211 (54) |
| Sentinel event, n (%) | 22/210 (10) |
| Full-term delivery, n (%) | 69/212 (33) |
| Instrumental labour, n (%) | 68/212 (32) |
| Caesarean, n (%) | 72/212 (34) |
| Apgar at 5min <5, n (%) | 40/210 (19) |
| Apgar at 10min <5, n (%) | 16/134 (12) |
| Meconium-stained amniotic fluid, n (%) | 75/202 (37) |
| Advanced resuscitation, n (%)a | 73/210 (35) |
| Umbilical cord pH≤7, n (%) | 111/156 (71) |
| Moderate or severe HIE in first 6h of life, n (%) | 33/213 (15) |
| Hypothermia treatment n (%) | 31/33 (94) |
| Extramural hypothermia treatment, n (%) | 22/31 (71) |
Of the 33 NBs with moderate/severe HIE, 31 were treated with hypothermia (94%).
Two were not, for the following reasons: in one case the grading of HIE was not carried out in time and in another the patient was not transferred because of instability secondary to severe pulmonary hypertension. Nine (9/31, 29%) were not transferred because they were born in one of the two hospitals with therapeutic hypothermia. Of the 22 that were transferred, 17 (77.3%) reached the receiving hospital within the first 6h of life, with a median of 5h (IQR, 1h). Passive hypothermia was initiated in all the NBs in their sending hospital; the mean temperature of the transferred infants on arrival at the receiving hospital was 33.1±1.2°C (range, 29–34.9°C). In the five NBs who reached the receiving hospital more than 6h after birth, the treatment was maintained, because hypothermia was initiated at their sending hospital within the 6h.
Ten infants (10/33; 30.3%) died during the programme. In 7/10 cases this was mainly related to the severity of the HIE, and of the remaining three one had a ruptured bowel, due to traumatic delivery, in addition to HIE, another had complex congenital heart disease and the third died from a neuromuscular disease.
DiscussionNeonatal encephalopathy due to perinatal hypoxic-ischaemic insult causes high neonatal morbidity and mortality in NBs greater than 35 weeks gestation, and those that survive the neonatal period have a high risk of serious and permanent lifelong consequences. Total body cooling or selective head cooling has proved to be an effective and safe therapeutic intervention for reducing mortality and major disability in survivors.8 Maximum therapeutic effectiveness is obtained when it is initiated as early as possible, and always within the first 6h of life.
Various conditions reduce the likelihood of appropriate care being provided in these first hours and of therapeutic hypothermia treatment being initiated in this narrow time frame. The most important of these are the following: (a) most newborns who develop HIE are born in hospitals with no neonatal intensive care unit or established hypothermia programme; (b) identifying the severity of HIE in these first hours of life is not easy and requires experience and clinical training; (c) certain comorbid conditions that can aggravate brain damage during those first hours need to be monitored, and (d) if the patient needs to be transported to a hospital with a hypothermia programme, this must be done urgently and under strict control.
This is why it is argued that a rapid and well-organised plan of action needs to be established within a few precious hours.4,5,9 To achieve this requires developing programmes that involve joint action between levels I and II neonatal units and medical emergency coordination centres (transport teams) with level III units, which offer integrated care, including hypothermia, for NBs with HIE.10–14 This joint plan of action of centres at various health care levels with transport services has been called the “hypothermia code”.4,9,15
The ARAHIP programme was designed specifically to establish this “hypothermia code”, and thereby organise and systematise care of NBs with perinatal hypoxic-ischaemic insult on a coherent basis. The programme sought, above all, to provide and safeguard the care these NBs need, and to reduce delays in initiating hypothermia treatment. One of its strengths is that it involves an extensive area of health care facilities (approximately 91000m2) and 12 hospitals, with neonatal units at various health care levels.
Although specific recommendations and programmes in the area of hypothermia treatment do exist,5,16–19 it is not easy to find protocols or clinical pathways that organise the whole process of the care of NBs at risk of developing HIE before therapeutic hypothermia is initiated.10,20 Hypothermia treatment needs to be strictly conducted in clinical practice in order to optimise its success outside clinical trials,21 and similar strictness is also required in prior monitoring and appropriate selection of candidates for receiving this treatment.10,22 In Spain, protocols have been developed at hospital level for the care of BNs during hypothermia treatment,23,24 but as far as we know the only existing programme similar to ARAHIP is the Hipocat programme in Catalonia. The ARAHIP programme, however, also offers a specific clinical pathway for the care, selection and early identification of NBs with hypoxic-ischaemic insult from birth in a large population area.
The incidence of one per 1000 live NBs detected in the programme is practically double that reported in two tertiary hospitals in Spain, one in Madrid and another in Barcelona.24,25 Our programme used the same definition of HIE and the same grading system as these hospitals, but our incidence is population-based, and therefore it is not limited to level III hospitals but has the virtue of including centres at different health care levels and a diverse range of hospitals, illustrated by the fact that only one of the centres has a neonatal service operating 24h a day. On the other hand, although there was only one case in which the severity of the encephalopathy was not correctly identified within the window period, it is quite possible that without the monitoring undertaken as part of the programme this number would have been higher. This highlights the need to establish monitoring programmes, training the professionals who attend deliveries to recognise HIE and the possible need for therapeutic hypothermia treatment.9,10,22 If we want to offer high quality care programmes delivered by expert teams with the appropriate technological means, it is essential to focus resources and rationalise the development of hypothermia programmes, which means that patients and programmes have to be centralised in the tertiary hospitals in each geographical area.4 The ARAHIP programme has not received any specific institutional support and it arose exclusively from collaboration and agreement among the professionals caring for these children.
Another of the programme's basic principles was checking for aggravating factors and for complications associated with hypoxic-ischaemic insult during the first 6h after birth.26–29 Although the programme does not address the management of neonates during the period of therapeutic hypothermia, it is very similar in the two hospitals that offer this therapy.
One of the limitations of the ARAHIP programme is the non-availability of a specialised transport service, although the programme itself made it possible to limit the consequences of this deficiency. One of the main obstacles to therapeutic success is arrival at the receiving hospital without hypothermia and outside the window period.16 The NBs in the ARAHIP programme reached the receiving hospital at a median of 5h and 91% of them with a temperature of around 34°C, which to a certain extent reflects the success of the programme. However, although the median temperature on arrival at the receiving hospital was 33.1°C, in 50% of cases it was below 33°C. Although the overcooling was slight, it can occur with passive hypothermia during transport,30 and our data are consistent with those reported in regions of similar size in other countries.11,31 Many of these regions have specialised transport, as well as servo-controlled cooling equipment, a preferable system for maintaining a stable temperature.32 In Spain, autonomous communities such as Madrid and Catalonia have trained teams with protocols for managing NBs with HIE during transport. The ARAHIP programme has made it possible, through systematic monitoring of every NB at risk of HIE and application of recommendations for transfer formulated as a protocol, to achieve a high rate of hypothermia treatment in the window period, initiated at the sending hospital and maintained during transfer.17,18 The excellent communications between the sending and receiving hospitals, with the support of the transport teams, has been a key factor in achieving these results. The fact that the NB is sometimes transferred by a neonatologist from the sending hospital may also have played an important role.
To sum up, the ARAHIP programme has made it possible to offer integrated care to NBs with possible perinatal hypoxic-ischaemic insult in the first hours of life by following a clinical pathway that includes specific protocols aimed at early identification of those with HIE, checking for factors that could aggravate brain injury, urgent transfer to a hospital with a therapeutic hypothermia programme and initiation of hypothermia within the time frame of the first 6h of life. Increasing experience among the professionals caring for these children, extended to as high a proportion of health care centres as possible, can only contribute to ensuring that these patients are offered the best care.
Conflicts of interestThe authors have no conflicts of interest to declare.
To Dr Amaia Cilla, for her contributions to the writing of the manuscript.
To the paediatric residents and nurses in the neonatal units, for their active involvement in carrying out the programme.
- –
Hospital Universitario, Burgos: María Miranda, Carmen Bustamante, Susana Schuffelmann, Cristina de Frutos and Joaquín Suárez.
- –
Hospital Santa Bárbara, Soria: Ruth Romero and Ana Peña.
- –
Hospital General, Segovia: Santiago Calleja.
- –
Hospital Nuestra Señora de Sonsoles, Ávila: Felipe Rubio, Ana María Jiménez, Manuel Felipe Marrero, Antonio Javier Martín and Sara Rupérez.
- –
Hospital Universitario, León: Daniel Mata, Maria Fernández and Lara García.
- –
Hospital Universitario, Salamanca: Ana Belén Remesal and Rubén García.
- –
Hospital General, Zamora: Victor Manuel Marugán.
- –
Hospital El Bierzo, Ponferrada: Rosario Velasco.
- –
Hospital Universitario Río Hortega, Valladolid: Sara Marín, Mar Montejo, Carla Escribano, Raquel Izquierdo, Elena Infante and María Samaniego.
- –
Hospital Santiago Apóstol, Miranda de Ebro: Ana Vereas.
- –
Hospital San Pedro, Logroño: María Beatriz Fernández and María Yolanda Ruiz.
Please cite this article as: Arnáez J, Vega C, García-Alix A, Gutiérrez EP, Caserío S, Jiménez MP, et al. Programa multicéntrico para la atención integral del recién nacido con agresión hipóxico-isquémica perinatal (ARAHIP). Anal Pediatr (Barc). 2015;82:172–182.
The ARAHIP Group is presented in Appendix A.
This study was presented in part at the following conferences: XXIV Congreso de Neonatología y Medicina Perinatal de la Sociedad Española de Neonatología, October 2013, Barcelona. 53rd Annual Meeting of the European Society for Paediatric Research, October 2013, Porto. XXIII Congreso de Neonatología y Medicina Perinatal de la Sociedad Española de Neonatología, October 2011, Oviedo. I Reunión entre Neonatólogos de Castilla y León, November 2011, Tordesillas. Reunión Primavera de la SCCALP, April 2011, Zamora.