The aim of this research is to contribute to the knowledge of the normal spontaneous motor behaviour of the human foetus during the second trimester of pregnancy. This study focuses on five patterns of spontaneous foetal movement: startle (S), axo-rhizomelic rhythmia (ARR), axial stretching (AS), general movement (GM), and diaphragmatic contraction (DC).
MethodsA cohort of 13 subjects was followed up using 2D obstetrical ultrasound images at 12, 16, 20, and 24 weeks of gestation. As inclusion criteria, neonatal neurological examination and general movements after eutocic delivery at term were normal in all of the subjects, and their neuromotor and cognitive developments until the end of pre-school age were also normal.
ResultsAll these five motor patterns are present at the beginning of the 2nd gestational trimester, but their quantitative and qualitative traits are diverse according to gestational ages. The phasic, isolated or rhythmically repeated movements, S and ARR, are prominent at 12 and 16 weeks of gestation, and then their presence gradually diminishes. By contrast, tonic and complex AS and GM movements increase their presence and quality at 20 and 24 weeks. RAR constitute a particular periodic motor pattern not described in previous literature. Moreover, the incidence of DC is progressive throughout the trimester, in clusters of 2–6 arrhythmic and irregular beats. Foetal heart rate increases during foetal motor active periods.
ConclusionsAll five normal behavioural patterns observed in the ultrasounds reflect the progressive tuning of motor generators in human nervous system during mid-pregnancy.
El objetivo de esta investigación es contribuir al conocimiento de la conducta motora fetal humana espontánea normal durante el 2.° trimestre de gestación. Se focaliza sobre 5 patrones de movimiento fetal: sobresaltos masivos (SM), ritmias axo-rizomélicas (RAR), estiramientos axiales (EA), movimientos generales (MG) y excursiones diafragmáticas (ED).
MétodosSe ha observado la motricidad fetal espontánea, mediante ecografía obstétrica en 2D, en una cohorte de 13 sujetos, en las semanas 12, 16, 20 y 24 de gestación. Constituye criterio de inclusión comprobar posteriormente la normalidad del estado neurológico neonatal a término y del desarrollo motor y cognitivo hasta la edad de 5 años.
ResultadosLos 5 patrones de movimiento citados se observan en todos los fetos durante el 2.° trimestre gestacional, pero su presencia y cualidad varían con la edad. Los movimientos fásicos SM y RAR son prominentes en las semanas 12 y 16 de gestación; en cambio, los movimientos prolongados EA y MG poseen mayor incidencia, duración, extensión y complejidad en las semanas 20 y 24. Las ED aumentan su incidencia a lo largo del 2.° trimestre, generalmente en series de 2-6 excursiones, con amplitud irregular. El ritmo cardiaco se acelera durante los periodos de movimiento fetal, frente al estado de reposo.
ConclusionesLos 5 patrones de conducta estudiados ecográficamente reflejan el progresivo afinamiento de generadores de patrones motores en el sistema nervioso humano normal durante el 2.° trimestre de gestación. Llamamos la atención sobre las RAR, no diferenciadas en otros estudios.
Starting at the end of the second month of gestation, the human embryo performs spontaneous movements classified by Reinold1 in 1979 into two broad categories: phasic and tonic-complex. Other researchers2–6 have identified movement patterns that become increasingly stable and recognisable from the end of the first trimester to the end of gestation,6,7 facilitating the development of a prenatal neurological semiotics and adding to morphological data and haemodynamic variables in the assessment of foetal wellbeing.7,8 Qualitative patterns are similar in foetuses of the same gestational age if their neurological status is normal.4,9–12 Foetal motor behaviour in the third trimester of gestation has been confirmed by extrauterine observation of preterm newborns.13
The aim of this study was to contribute additional information on the normal developmental characteristics of five prominent types of spontaneous foetal movement in the second trimester of gestation by the visual analysis of 2D obstetric ultrasound images.
Subjects and methodsSubjectsWe performed a prospective cohort study of 13 foetuses whose mothers, aged between 20 and 38 years and with no risk factors, consecutively sought prenatal care for a normal pregnancy starting at the eight postmenstrual week in the Department of Obstetrics and Gynaecology of our hospital in 2005. The project was approved by the ethics board of the hospital, and the mothers signed the informed consent form after being told that second-trimester monthly ultrasound explorations would be prolonged by 30min to analyse foetal movements and that this was a harmless procedure, adhering to the criteria of the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG).14,15 Every foetus in the cohort (seven females and six males) was born to term in an eutocic delivery and had a normal weight, length, and head circumference and an Apgar test score ranging from 9 to 10 measured between the first and fifth minute after birth. On the third day after birth, the spontaneous behaviour of every newborn while awake and not crying included a type of general movement known as writhing movements,16,17 and the rest of the conventional neurological examination 18–21 was normal in all subjects. Regular paediatric follow-up visits until 5 years of age confirmed that all 13 subjects had normal psychomotor and language development and academic performance.
MethodsTransabdominal ultrasonography was performed with a Voluson 730 machine (G.E. Healthcare, Milwaukee, USA) with a 2-5MHz probe. The morphological examination was done using the 4D mode (movement on three planes), and the foetal wellbeing parameters and spontaneous movements were assessed using the 2D mode (movement on one plane) at weeks 12, 16, 20, and 24 of gestation. The machine scans 40 frames per second in 2D mode, which allowed the observation of foetal movements with no breaks in continuity. The safety indices used were a mechanical index of 0.9 and a thermal index of 0.1, as recommended by the ISUOG.14,15 All examinations were performed 3h following the last intake of food by the mother, at an environmental temperature between 23°C and 26°C and in the absence of extraneous noises or interferences. The analogue U-Matic recordings were digitised. A single obstetric ultrasonographer (JLA) acquired all the material, while two neuropaediatricians (CR and JN) performed the visual analysis of the recordings of foetal movements.
We defined the applicable observation time (OT) of foetal activity as the period of the 2D ultrasound during which the ultrasound image contained a full parasagittal plane of the foetal body without external stimulation (Table 1). We documented the heart rate of the foetus during periods of motor activity and periods of quiescence. Following an initial examination of the material, we decided to limit our analysis to the five movement patterns that were most prominent:
- –
Startle (S): a sudden and isolated extension of the trunk in the cephalocaudal direction lasting between 1 and 3s and without active movement of the extremities.2,9
- –
Axo-rhizomelic rhythmias (ARRs): excursions along the cervicodorsal axis that travel symmetrically along the proximal portions of the four extremities, occurring in rhythmic clusters.
- –
Axial stretching (AS): tonic posture in axial extension held for two or more seconds; it may be restricted to the cervical region or extend to the rest of the body axis, with negligible movement of the limbs.4
- –
General movements (GMs): complex movement patterns that combine flexions, extensions and rotations, with the asynchronous involvement of different parts of the trunk and extremities and of varying speed, amplitude, and direction.2,7,10 The morphology of GMs is fluid and unpredictable, and their complexity and variety increase during the second and third trimesters. MGs continue to appear in waves with increasing duration, complexity and variability through the second month post-term during quiet wakefulness and REM sleep.10,13,16–18
- –
Diaphragmatic contractions (DC): series of contractions of the diaphragm, irregular in speed and rhythm, in clusters of 1–10 beats. Other published works refer to these as “hiccups” and “breathing movements”,2,5,6,9,10 but since they do not have those functions in the foetus, we have interpreted them phenomenologically as practising diaphragmatic movements.
Characteristics of foetal movements at four times during the second trimester of gestation (weeks 12, 16, 20 and 24) and in the full-term newborn: presence of movement; number and incidence of events; duration; amplitude of movement (short or long); and rhythm of movement (isolated or clustered).
| Weeks of gestation | Newborn (NB) | |||||
|---|---|---|---|---|---|---|
| 12 | 16 | 20 | 24 | |||
| OT: applicable observation time in minutes: mean (SD) | 22.1 (6.1) | 14.2 (3.5) | 14.9 (4.8) | 13 (1.7) | ||
| MT: time spent in movement, in minutes: mean (SD) | 6.4 (2) | 3.2 (0.8) | 2.8 (0.8) | 1.99 (0.8) | ||
| % Time spent in movement (MT) relative to OT | 29.69% (7.5) | 23.77% (7.3) | 19.69% (4.9) | 15.3% (5.9) | ||
| TMT: time spent in target movements: mean (SD) | 2.68 (1.21) | 2.39 (0.8) | 1.58 (0.7) | 1.38 (0.4) | ||
| % Time spent in target movements (TMT) relative to MT | 76.5% (23.5) | 74.1% (32.8) | 59.36% (29.1) | 80.4% (39.7) | ||
| Startles (S) | Presence: N participants | 13 | 10 | 3 | 0 | 0 |
| -Total number of events per participant: mean (SD) | 29 (13.7) | 19 (4.7) | 5.3 (1.5) | – | ||
| -Incidence of events per participant: mean (SD) | 13.8 (6.3) | 14.6 (4.8) | 3.1 (0.6) | – | ||
| -time per participant (seconds): mean (SD) | 32.15 (14.98) | 19.21 (5.71) | 7.11 (2.14) | – | ||
| % time relative to OT | 2.53% (1.14) | 2.38% (0.9) | 0.86% (0.3) | – | ||
| % time relative to MT | 9.31% (5.8) | 10.35% (4.2) | 4.56% (1.9) | – | ||
| Amplitude: short/long | 2/11 | 2/8 | 1/2 | – | ||
| Rhythm: isolated/clustered | 13/0 | 5/5 | 0/3 | – | ||
| Axo-rhizomelic rhythmias (ARR) | Presence: N participants | 11 | 6 | 6 | 3 | 0 |
| -Total number of events per participant: mean (SD) | 2.5 (0.8) | 1.7 (0.5) | 1.5 (0.5) | 1 (0) | ||
| -Incidence of events per participant: mean (SD) | 1.2 (0.5) | 1.3 (0.4) | 1.1 (0.5) | 0.8 (0.1) | ||
| -time per participant (seconds): mean (SD) | 14.54 (8.31) | 4.62 (5.56) | 3.08 (4.35) | 0.92 (1.75) | ||
| % time relative to OT | 1.15% (0.7) | 0.58% (0.7) | 0.36% (0.6) | 0.12% (0.6) | ||
| % time relative to MT | 4.17% (2.6) | 2.53% (3.2) | 1.81% (2.7) | 1.09% (2.4) | ||
| Amplitude: short/long | 11/0 | 6/0 | 4/2 | 2/1 | ||
| Rhythm: isolated/clustered | 11/0 | 6/0 | 2/4 | 0/3 | ||
| Axial stretching (AS) | Presence: N participants | 3 | 6 | 11 | 12 | 13 |
| -Total number of events per participant: mean (SD) | 3.3 (1.6) | 5.2 (2.1) | 6.1 (2.6) | 2.2 (0.9) | ||
| -Incidence of events per participant: mean (SD) | 3.2 (1) | 4.5 (2.1) | 4.3 (2.1) | 1.7 (0.8) | ||
| -time per participant (seconds): mean (SD) | 0.77 (2.77) | 14.31 (18.1) | 22.31 (19.11) | 14.54 (8.31) | ||
| % time relative to OT | 0.05% (0.2) | 2.09% (2.6) | 2.67% (2.4) | 1.9% (0.5) | ||
| % time relative to MT | 0.12% (0.4) | 7.86% (10.8) | 14.04% (11.7) | 12.5% (3.8) | ||
| Amplitude: cervical/whole trunk | 3/0 | 1/5 | 1/10 | 8/4 | ||
| Rhythm: isolated/clustered | 3/0 | 6/0 | 10/1 | 11/1 | ||
| General movements (GM) | Presence: N participants | 10 | 13 | 10 | 10 | 13 |
| -Total number of events per participant: mean (SD) | 5.6 (3.7) | 5.5 (1.5) | 4.9 (2.2) | 2 (0.7) | 1.9 (0.5) | |
| -Incidence of events per participant: mean (SD) | 5 (1.8) | 4.4 (1.4) | 3.6 (2.1) | 1.6 (0.5) | 1.3 (0.4) | |
| -Time per participant (seconds): mean (SD) | 113.2 (40.9) | 103.5 (20.5) | 58.8 (26.9) | 32.5 (11.9) | ||
| % Time relative to OT | 13.36% (5) | 12.28% (5.6) | 5.55% (2.9) | 3.56% (2.2) | ||
| % Time relative to MT | 62.9% (19.4) | 52.22% (21.7) | 28.43% (22.8) | 27.78% (22.3) | ||
| Amplitude: short/long | 5/5 | 10/3 | 10/0 | 0/10 | 2/11 | |
| Rhythm: isolated/clustered | 7/3 | 11/2 | 10/0 | 10/0 | 13/0 | |
| Diaphragmatic contractions (DC) | Presence: N participants | 3 | 11 | 12 | 12 | 13 |
| -Total number of events per participant: mean (SD) | 1 (0) | 1.3 (0.5) | 2.7 (0.9) | 4.3 (1.1) | ||
| -Incidence of events per participant: mean (SD) | 0.3 (0.1) | 0.9 (0.5) | 1.9 (0.6) | 3.3 (0.7) | ||
| -time per participant (seconds): mean (SD) | 0.9 (0.1) | 1.85 (3.51) | 17.23 (7.89) | 36 (12.73) | ||
| % time relative to OT | – | 0.26% (0.5) | 2% (1.1) | 4.6% (1.8) | ||
| % time relative to MT | – | 1.14% (0.05) | 10.53% (5.6) | 34.94% (10.7) | ||
| Amplitude: short/long | 3/0 | 11/0 | 12/0 | 12/0 | 13/0 | |
| Rhythm: isolated/clustered | 1/2 | 0/11 | 0/12 | 0/12 | 13/0 | |
Any observed occurrence of these five movement patterns at 12, 16, 20, and 24 weeks of gestation (total number of events) was recorded in the data sheet, and we calculated their incidence (mean number of events per 10min of observation) for the entire series for each point in time. We calculated the total time during which foetal movements were observed, as well as the time spent in each type of movement and on all the five target movements combined. We classified movements as isolated or clustered depending on their rhythm, and as short or long based on the amplitude of the displacement (Table 1).
Statistical analysisWe conducted a longitudinal study with repeated measures allowing for the description of foetal movement patterns through the four observation dates during the second trimester of gestation and the term neonatal period by means of a mixed linear model. We used the SPSS 10.0 software to calculate the descriptive statistics of quantitative variables for each movement pattern and did a repeated measures ANOVA to compare the patterns at different gestational ages. The nominal variables that characterise foetal movements were analysed using the Friedman test.
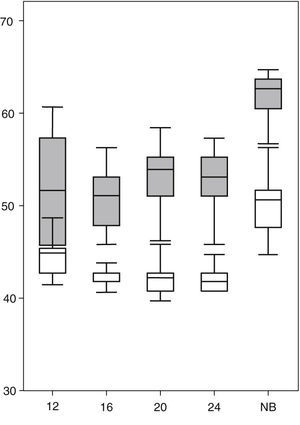
ResultsAs shown in Table 1, the percentage of the OT during which foetuses displayed some kind of movement decreased significantly across the four successive examinations from week 12 to week 24. The five target movement patterns that this study focused on amounted to between 60% and 80% of the total motor activity of each foetus. The variables pertaining to the presence and characteristics of each of the five behaviours under consideration in the study during the second trimester of gestation and the term neonatal period are summarised in Table 1. In this series, the foetal heart rate percentile22 increased significantly during motor activity periods compared to periods of quiescence (Fig. 1).
Heart rate percentiles (ordered) of the 13 foetuses in quiescence (white) and movement (grey). We found significant differences (P<.05) at the four observation times of the second trimester of gestation (weeks 12, 16, 20 and 24) and the term neonatal period (NB), marked in the horizontal axis.
Foetal movement was observed on a mean 29.7% of the OT. Startles were the most frequent movements among the five target-patterns of our study, as they occurred in every foetus at a rate of 13.8 startles per 10min. They usually appeared clustered in arrhythmic clusters of 2–15 successive startles; sometimes a cluster of startles was followed by a GM. ARRs were observed in 11 foetuses and amounted to 4.17% of the total motor activity time. Isolated AS movements restricted to the cervical region were observed in three foetuses. GMs were clearly discernible in 10 foetuses, with a mean incidence of five events per 10min, amounting to 13.3% of the OT. The presence of DCs was only observed in three foetuses; they were short in amplitude and occurred in isolation or in clusters.
Week 16 of gestationThere was evidence of foetal movement for a mean 23.8% of the total OT. Motor behaviour was more organised, as movements were frequently combined, with one leading to another. The incidence and duration of AS, GMs, and DCs increased; the latter usually had a short amplitude and occurred in clusters. In contrast, the observed presence of the simpler and more abrupt movements, startles and ARRs, started to decline (Table 1). The most prevalent events were GMs, as they were observed in every foetus and amounted to 12.3% of the OT. The presence of ARRs decreased by half, with very few events per foetus.
Week 20 of gestationMotor activity became slower and more complex, as simpler and more abrupt movements, like startles and ARRs, decreased dramatically in their presence and incidence, while the considerable presence of AS and GMs was sustained, with these patterns accounting for most of the foetuses’ motor activity, amounting to 19.7% of the OT. Both AS and GMs were observed in eight foetuses; AS was observed in three foetuses that did not have any GMs at this time point; conversely, GMs were observed in two foetuses in whom no AS was detected at this age; while it was only in one foetus that neither GMs nor AS was detected, although they were detected in the preceding and following examinations. Clusters of AS were clearly perceived in 12 foetuses.
Week 24 of gestationThe five target movements accounted for 80% of the total foetal motor activity at this age. The incidence of all patterns decreased compared to previous weeks, but the behaviour patterns increased in complexity and duration. Startles were disappearing and ARRs were only observed in three foetuses. Meanwhile, AS and DCs were observed in 12 foetuses and amounted to 1.9 and 4.6% of the OT, respectively. Compared to the preceding gestational weeks, the patterns with the highest incidence at this age were GMs and DCs, which accounted for 27.8% and 35% of the total foetal motor activity, with characteristics that were similar to those observed at earlier ages.
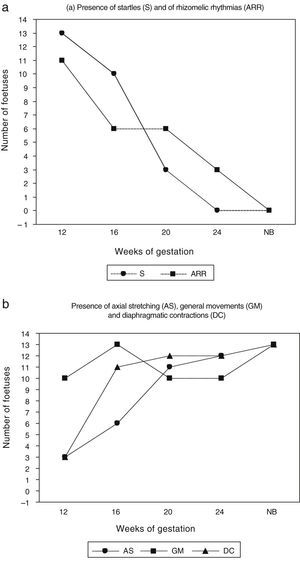
Differential chronology of the five target movements under studyIt is apparent from Fig. 2 and Tables 1 and 2 that the shortest and fastest foetal skeletal movements (S and ARRs) were observed very frequently in week 12 and then gradually decreased until they practically disappeared by week 24. In contrast, the presence of slow, prolonged and arrhythmic movement patterns involving the trunk increased gradually. Thus, AS was observed in an increasing number of foetuses throughout the second trimester of gestation. GMs were observed in most foetuses at 12, 20 and 24 weeks of postmenstrual age, and were seen in the entire sample at week 16 (Fig. 3).
(a) Presence of startles and axo-rhizomelic rhythmias at the four observation times in the second trimester of gestation (12, 16, 20 and 24 weeks) and the term neonatal period (NB). (b) Presence of axial stretching, general movements and diaphragmatic contractions at the four observation times in the second trimester of gestation (weeks 12, 16, 20 and 24) and the term neonatal period (NB).
Differences in the presence and incidence of events and time spent in movement at the four observation times.
| Foetal movements | Presence | Incidence of events | Time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 16 | 20 | 24 | 16 | 20 | 24 | 16 | 20 | 24 | |
| Startles | |||||||||
| 12 | n.s. | ** | ** | n.s. | ** | ** | * | ** | ** |
| 16 | * | ** | ** | ** | * | ** | |||
| 20 | n.s. | n.s. | ** | ||||||
| Axo-rhizomelic rhythmias | |||||||||
| 12 | n.s. | n.s. | ** | n.s. | n.s. | ** | * | ** | ** |
| 16 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| 20 | n.s. | n.s. | n.s. | ||||||
| Axial stretching | |||||||||
| 12 | n.s. | ** | ** | n.s. | ** | ** | n.s. | * | ** |
| 16 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| 20 | n.s. | n.s. | n.s. | ||||||
| General movements | |||||||||
| 12 | n.s. | n.s. | n.s. | n.s. | n.s. | * | * | * | * |
| 16 | n.s. | n.s. | n.s. | * | * | * | |||
| 20 | n.s. | n.s. | n.s. | ||||||
| Diaphragmatic contractions | |||||||||
| 12 | * | * | * | ** | ** | ** | n.s. | ** | ** |
| 16 | n.s. | n.s. | * | ** | ** | ** | |||
| 20 | n.s. | * | ** | ||||||
Statistical analysis method: repeated measures ANOVA and Friedman test.
n.s.: no significant difference.
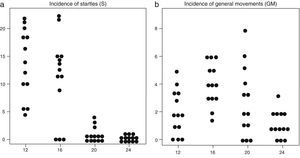
(a) Incidence of startle events (in vertical axis) in the 13 foetuses at the four observation times in the second trimester of gestation (in horizontal axis: weeks 12, 16, 20 and 24). (b) Incidence of general movements (in vertical axis) in the 13 foetuses at the four observation times in the second trimester of gestation (in horizontal axis: weeks 12, 16, 20 and 24).
Consistent with the above, the percentage of time spent in startles and ARRs declined from week 12 throughout the second trimester of gestation, while the time spent in episodes of AS increased from week 20 in comparison to week 12. GMs occurred in most foetuses at the four observation times, but motor events had a shorter duration as gestational weeks passed. The mean incidence of motor events was lower at week 24 compared to preceding weeks.
Diaphragmatic contractions were observed rarely at week 12, but they were detected in 11 foetuses from week 16, and amounted to an increasingly longer portion of the observation time through the end of the second trimester. Diaphragmatic movements usually occurred in clusters of several arrhythmic excursions of short amplitude; they frequently appeared as diaphragmatic flutters or “hiccups”.
DiscussionThe results of our study show that from the start of the second trimester extended and complex movements such as AS and GMs occur prominently, with the second of these two constituting the most elaborate form of foetal movement, starting, according to other studies, late in the first trimester,4,6,12 and remaining in their richest forms through the first two months post-term.10,13,16,17 In our study, GMs were observed in all foetuses starting at week 16, and in 10 of them at week 12, although other authors9–12 have reported their sustained presence in their series starting at week 9 using longer periods of observation of up to 60min. On the other hand, the abrupt movements, startles and ARRs, which exhibit a strong presence among the behavioural patterns in the early second trimester of gestation, proceed to disappear gradually.
The different patterns of motor behaviour in human foetal motor activity depend on central pattern generators (CPGs) in the nervous system.23–25 The neural oscillatory activity generated at different levels of the spinal cord and the brainstem has been investigated in experimental studies of nonhuman vertebrates (lamprey, zebrafish, salamander, chicken, mouse, sheep, monkey), and is associated with different forms of locomotion and other rhythmic activities that make breathing, ingestion, and swallowing possible.23,24 Each individual has a genetic repertoire for the formation of neural networks; the histogenesis of the nervous system also involves intercellular chemotactic factors, growth factors in the glia-neuron network, and hormonal substances secreted by the endocrine systems of the foetus and the mother.25,26. The propioceptive feedback generated by the motor activity of the foetus and the tone-modulating influences from the reticulospinal, vestibulospinal, tectospinal and rubrospinal tracts on the spinal cord fine-tune spontaneous motor behaviour through action on the spinal CPGs.27 Meanwhile, the long descending motor tract axons, which are eventually controlled by the basal ganglia and the cerebellum,28 originate at the cortical subplate and develop through the third trimester of gestation. In man, the corticospinal tract, still unmyelinated, reaches the caudal medulla at 8–9 weeks of postmenstrual age and completes decussation by 15 weeks; later on, the corticospinal axons reach the cervical level at 17 weeks, the thoracic level at 19 weeks, and the lumbosacral level at 29 weeks of gestation.29–32 All these suprasegmental influences progressively select and coordinate the different rhythmogenic structures of the brainstem and spinal cord, increasing the complexity and purpose of movements.26,27
Thus, since the four patterns of foetal skeletal movements analysed in this study (S, ARR, AS and GM) occur prior to the onset of corticostriatal modulation, it is possible that they originate in the spinal cord and brainstem generators. A study that observed a series of anencephalic foetuses33 described a higher frequency of startles and GMs than that found in healthy foetuses, and abnormal quality of motor activity in its excessive amplitude, jerkiness, and poor repertoire. Since rostral action potentials originating in the telencephalic plate influence the characteristics of GMs, the latter are altered or missing when there is an early pathology of the corticospinal control developed during gestation or the perinatal period (periventricular leukomalacia, subcortical leukomalacia, etc.).16,17
The ARRs, which in our cohort were most frequently observed in weeks 12 and 16 of gestation, constitute a good example of global rhythmic motor activity that is synchronous in both sides of the body. The presence of both ARRs and startles starts to decline in the second half of the second trimester. Quick and global movement patterns have also been found in the first half of gestation in other mammals (mouse, sheep), in which the movements that repeat periodically and simultaneously in both sides of the body or alternating either side as a prelude to locomotion persist after transection of the high spinal cord34,35 because they are generated in autonomic centres of the spinal cord. In man, tonic and broad movement patterns, such as AS and GMs, persist throughout the second and third trimesters under the control of the brainstem and spinal cord27. Rostral action potentials originating in the telencephalic plate influence the characteristics of GMs, which are altered or absent when there is an early pathology of the corticospinal system.
The diaphragmatic contractions present in the second trimester of gestation are reminiscent of the diaphragmatic flutter observed in acquired pathologies of the high cervical cord36 or the sigh-like (‘ataxic’) breathing pattern of advanced rostrocaudal deterioration.37 During the last trimester of gestation, DCs become increasingly efficient in displacing amniotic fluid,2,9 and upon contact with the air at birth they adopt the cyclical automatic rhythm of breathing generated in the pacemakers next to the Bötzinger complex.38,39
There is evidence that the interobserver reliability is high for investigators well trained in Prechtl's observational method for assessing spontaneous movement in preterm and term newborns and young infants.40 A limitation of our study, which it shares with other exploratory works, is the small number of foetuses and, considering the broad interindividual variability of motor behaviour, the relatively brief observation time. Our observations, together with those of other studies with similar cohorts and methodologies, may contribute to refining our knowledge of normal foetal motor patterns in the second trimester of gestation.
FundingThis study received funding from a grant for research in Developmental Neurology of the Fundación Fuentes Dutor-ICT Pamplona.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Reynoso C, Crespo-Eguílaz N, Alcázar JL, Narbona J. Motricidad fetal durante el segundo trimestre de gestación: estudio ecográfico longitudinal. An Pediatr (Barc). 2015;82:183–191.