X-linked agammaglobulinemia (XLA) is characterised by an arrest of B cell differentiation, leading to recurrent bacterial infections. Lifelong immunoglobulin replacement therapy (IRT) is indicated to prevent infections and their complications.
Materials and methodsA retrospective study of patients with XLA followed in a level three hospital was performed; data was collected retrospectively by review of clinical files.
ResultsXLA was diagnosed in 9 children. One (11%) had a positive family history with a prenatal diagnosis. Infection was the clinical presentation in all the others (89%), at an average age of 13 months; diagnosis was established at a mean age of 3.4 years. Acute otitis media (7/9) and pneumonia (5/9) were the most frequently observed. Seven (78%) presented serum immunoglobulin G (IgG) levels below 200mg/dL and all of them had CD19+ B cells below 2%. Neutropenia was present at diagnosis in three patients (33%). Bruton tyrosine kinase (BTK) mutations were identified in all cases. Intravenous IRT was initiated, switched later to subcutaneous administration, in all. The mean time of follow-up was 10.7 years with cumulative time of 97 years. Eight children (89%) achieved IgG serum levels above 800mg/dL. One presented lower values due to renal loss. No deaths occurred. After diagnosis the most frequent infections were acute otitis media (6/9). In spite of stable adequate IgG levels on IRT, two patients developed bronchiectasis.
ConclusionsXLA overall prognosis is good, as long as patients have an early and adequate treatment. However, bronchiectasis can occur even on adequate immunoglobulin replacement therapy.
La agammaglobulinemia ligada al cromosoma X (ALX) se caracteriza por la detención de la diferenciación celular de los linfocitos B, que da lugar a infecciones bacterianas recurrentes. La terapia de por vida de reemplazo de inmunoglobulina (TRI) está indicada para prevenir infecciones y sus complicaciones.
Material y métodosSe hizo un estudio retrospectivo de pacientes con ALX en un hospital terciario. Los datos se revisaron retrospectivamente revisando historias clínicas.
ResultadosSe diagnosticó ALX en 9 niños. Uno de ellos (11%) tenía antecedentes familiares y había sido diagnosticado prenatalmente. El resto presentaron signos de infección (89%) a una edad media de 13 meses, siendo su edad media al diagnóstico de 3,4 años de edad. Las infecciones más frecuentes fueron otitis media aguda (7/9) y neumonía (5/9). Siete niños (78%) presentaron niveles séricos de inmunoglobulina G (IgG) inferiores a 200mg/dL, y todos tenían niveles de células B CD19+ B por debajo del 2%. Tres pacientes tuvieron neutropenia al diagnóstico (33%). En todos los casos se detectaron mutaciones en la tirosina cinasa de Bruton (BTK). También en todos se inició la TRI por la vía intravenosa, que luego continuó por la vía subcutánea. La duración media del seguimiento fue de 10,7 años, con un número total acumulado de 97 años. Ocho niños (89%) alcanzaron niveles séricos de IgG superiores a los 800mg/dL. En un caso se observaron niveles más bajos por pérdida renal. No hubo ninguna defunción. El tipo de infección más frecuente tras el diagnóstico fue la otitis media aguda (6/9). A pesar de haberse conseguido niveles estables adecuados de IgG mediante la TRI, 2 pacientes desarrollaron bronquiectasias.
ConclusionesEn general, el pronóstico de la ALX es bueno siempre y cuando los pacientes reciban el tratamiento adecuado de manera precoz. Aún así, es posible que a pesar de ser tratados correctamente con TRI desarrollen bronquiectasias.
X-linked agammaglobulinaemia (XLA [MIM 300755]) is a primary immunodeficiency characterised by the arrest of B cell differentiation,1 leading to a considerably reduced B lymphocyte count and low serum immunoglobulin (Ig) levels that make patients more susceptible to recurrent and severe infections.2 The infections usually start appearing between 3 and 6 months of age, when maternal IgG levels start to decline. If there is a family history of the disease, XLA may be suspected and diagnosed prenatally.3 Respiratory tract infections by encapsulated bacteria, especially otitis, sinusitis, and pneumonia, are characteristic of XLA.2,4,5 Most of these infections are caused by encapsulated pyogenic bacteria (Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus).2 The literature shows that the pathogens isolated most frequently from patients with septicaemia correspond to various Pseudomonas species.2,6 Digestive tract infections by Giardia lamblia are also frequent.2 Patients with XLA are particularly vulnerable to enteroviruses, especially polioviruses and coxsackieviruses. There have been reports of poliomyelitis caused by administration of the live attenuated vaccine, associated with high mortality rates.2
The estimated incidence of XLA ranges from 1:100,000 to 1:200,000 cases per live births.2 The disease is caused by mutations in the BTK gene that encodes Bruton tyrosine kinase, located in the X chromosome (Xq21.3–Xq22).7 The BTK protein is involved in every stage of the development of the B cell lineage and in the myeloid and erythroid precursors, and does not affect T lymphocytes or NK cells. Mutations in this protein cause defects in the early stages of B cell development, leading to a considerable reduction in B cell blood levels.2 Over 800 different mutations have been described to date.8 The detection of mutations in the BTK gene is a necessary criterion for the definitive diagnosis of XLA and for genetic counselling.9
Lifelong immunoglobulin replacement therapy (IRT) is indicated for patients with XLA. If the disease is diagnosed early, patients can have a good quality of life. Early treatment with IRT is essential to reduce the recurrence and severity of infections, the number of hospital admissions, and morbidity due to chronic complications of the disease.2,10
The purpose of this study was to learn the characteristics of patients with XLA followed up at the paediatric infectious diseases and immunodeficiencies unit of the department of paediatrics of a tertiary hospital in northern Portugal, analysing their clinical presentations and outcomes.
Materials and methodsStudy design and protocol: we performed a descriptive retrospective study of the patients diagnosed with XLA and followed up at the paediatric infectious diseases and immunodeficiencies unit of the department of paediatrics of a tertiary hospital in northern Portugal between January 1991 and December 2013. We collected the data from the patients’ medical records.
Data: we collected data on the factors that led to the diagnosis (positive family history or presence of infections); age at clinical onset (based on the date of the first infection); age at diagnosis; type of infections developed prior to diagnosis and hospitalisations; IgG levels; CD19+ B cell number at diagnosis; identified BTK mutation; duration of IRT administration; and infections and non-infectious complications during followup.
Definitions used by the authors: the diagnosis of XLA was based on established criteria: male patient with fewer than 2% CD19+ B cells and mutation in the BTK gene, in accordance to the definition of the European Society for Immunodeficiencies.11
We considered that there was a family history of XLA if any family members had been diagnosed with it.
We defined delay in diagnosis as the time difference between the age at the onset of symptoms (first infection) and the age at diagnosis.
Recurrent otitis media was defined as the occurrence of 3 or more episodes of acute otitis media, and recurrent pneumonia as the occurrence of more than one episode of pneumonia.
Intravenous replacement therapy involved the intravenous administration of IgG every 3 or 4 weeks in the dosage needed to maintain minimum serum IgG levels above 800mg/dL, or the subcutaneous administration of IgG once or twice a week. The subcutaneous preparation has been available in Portugal since 2007.
Descriptive analysis: the results of the quantitative variables are expressed as mean, median, and range, and the results of categorical variables as percentages.
ResultsOf the 247 patients with antibody deficiencies followed up in our paediatric infectious diseases and immunodeficiencies unit, 9 (3.6%) were diagnosed with XLA (Table 1 presents their clinical and laboratory data).
Clinical presentation and laboratory data.
| Patient | Age at onset of symptoms (months) | Age at diagnosis (months) | Infections before diagnosis | Neutropaenia at diagnosis | Serum IgG (mg/dL) | CD19+ (% of total lymphocyte count) | BTK mutation | Complications during followup | Duration of IRT (years) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Infectious | Non-infectious | |||||||||
| 1 | 2 | 36 | Recurrent OM; otitis with mastoiditis; pneumonia with pleural effusion | No | 53 | 0.1 | p.Arg641His | Acute OM; conjunctivitis | 5 | |
| 2 | 2 | 16 | Recurrent OM; ecthyma gangrenosum | Yes | 43 | 0.1 | p.Tyr361X | Acute OM; conjunctivitis | 6 | |
| 3 | 24 | 48 | Recurrent OM; septic arthritis | No | 214 | 0.2 | c.1178-1G>A (intron 13) | Acute OM; giardiasis | 10 | |
| 4 | 9 | 72 | Recurrent pneumonia; recurrent OM; acute gastroenteritis | No | 720 | 0.6 | c.1178-1G>A (intron 13) | Acute OM; giardiasis | Bronchiectasis | 13 |
| 5 | – | Neonatal perioda | – | No | 0 | 1.4 | p.Arg288Gln | Pneumonia | Membranoproliferative glomerulonephritis | 12 |
| 6 | 35 | 42 | Recurrent OM; pneumonia | Yes | 0 | 0 | p.Glu7X | Acute OM; conjunctivitis; giardiasis | 5 | |
| 7 | 6 | 72 | Recurrent pneumonia; septic arthritis | No | 0 | 0 | Exon 6 deletion | Conjunctivitis; sinusitis | 22 | |
| 8 | 24 | 36 | Recurrent OM; pneumonia | Yes | 0 | 0.1 | Exon 16 deletion | Acute OM; conjunctivitis | Bronchiectasis | 13 |
| 9 | 2 | 12 | Recurrent OM | No | 0 | 0 | p.Arg255X | 11 | ||
OM, otitis media.
In most cases, the diagnosis stemmed from severe or recurrent infections (8–89%), with a mean age at diagnosis of 3.4 years (range, 1–6 years) and of 13 months at clinical onset (median, 7.5 months; range, 2–35 months). Most patients (63%) had clinical manifestations in the first year of life. Five cases (63%) were diagnosed before 3 years of age. The mean time elapsed from the onset of symptoms to diagnosis was 2.5 years. All of these patients had been hospitalised due to infections.
Respiratory infections were the most common type of infection before diagnosis, with otitis media, complicated by mastoiditis in one case, being the most frequent (88%). Pneumonias also occurred frequently (56%), and they were recurrent in 4 of the patients. Two patients had septic arthritis, and one was admitted to the hospital with ecthyma gangrenosum associated with a Pseudomonas infection. Table 1 shows the infections that occurred before diagnosis.
A patient with a family history of XLA was diagnosed prenatally and treated during the neonatal period before acquiring any infections.
Most patients had very low serum IgG levels at diagnosis, with a mean of 114mg/dL, with 7 patients (78%) having levels below 200mg/dL. The B cell counts ranged from 0% to 1.4%.
One third of the children had neutropaenia associated with infection. The minimum concentrations ranged from 0 to 510cells/mm3, and 2 children had neutrophil counts below 500cells/mm3. The values normalised in all three during the first week of treatment with antibiotics and IRT. Neutropaenia did not recur in any of them.
A different mutation of the BTK gene was identified in each case except in patients 3 and 4, who were siblings (Table 1).
All patients started IRT through the intravenous route (IVIg) to quickly reach adequate levels of IgG. Once this happened, all patients chose to switch to subcutaneous IgG (SCIg) to self-administer at home.
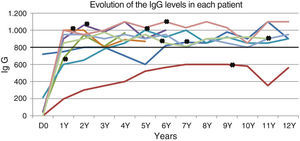
The mean duration of IRT was 10.7 years, with a cumulative total of 97 years. Eight patients reached adequate minimum levels of IgG; minimum concentrations above 800mg/dL could not be achieved despite high doses of replacement IgG in only one case (Fig. 1). This last patient developed a nephrotic syndrome at 10 years of age, with severe protein loss through urine.
We observed mild infectious complications—acute otitis media and conjunctivitis—in most patients after initiating IRT. None of them required admission to the hospital. Two patients developed bronchiectasis (patients 4 and 8).
There were no deaths. All patients have grown normally, and to date none of them have developed autoimmune diseases or cancer.
DiscussionThe current literature shows that about 50% of patients with XLA have clinical manifestations before one year of age, and that approximately 50% of cases are diagnosed before 2 years of age.3 The results of our study were similar on both aspects. Consistent with previous studies, the most common clinical presentation was infection (89%). Recurrent bacterial infections and lack of response to oral antibiotics were the main clues that led to the diagnosis of immunodeficiency. Recurrent otitis media was the most frequent infection among our patients before diagnosis, followed by pneumonia, which is consistent with the literature.12–14
Patient 1 had the classical presentation of XLA (Table 1), with recurrent episodes of acute otitis media starting at 2 months of age, 3 episodes of mastoiditis, and one bacterial pneumonia with pleural effusion at 3 years of age, which is when XLA was diagnosed. Patient 2 had an atypical presentation of XLA (Table 1): he was admitted to the hospital with septicaemia associated with Pseudomonas and ecthyma gangrenosum at 16 months of age. This presentation has been described previously in the literature, and may occur in 10% of cases.2
In our study, the mean age at diagnosis was 3.4 years, with a diagnostic delay of 2.5 years. Since a higher degree of suspicion may shorten the diagnostic delay, we should increase paediatricians’ awareness of this rare condition. Analysing serum Ig levels is cheap and easy. If the diagnosis is made earlier, treatment can be initiated earlier, leading to better outcomes.
One of the children benefited from a prenatal diagnosis that allowed the initiation of IRT before acquiring any infections, illustrating the importance of genetic tests to detect mutations in the BTK gene both for confirming the diagnosis and for detecting carriers and offering prenatal counselling to families.
The published data show that neutropaenia is found in patients with XLA in variable percentages that range between 10% and 25%.15,16 Neutropaenia was part of the clinical presentation of 11% of the cases in a United States registry before initiation of IRT,3 and was described in 18% of the cases in a nationwide study in Japan.17 While the role of the BTK gene in neutrophil development has yet to be determined, this gene is associated with high bacterial loads characteristic of active infections that usually respond to treatment with antibiotics and Ig.16 Our data showed neutropaenia in one third of the cases (patients 2, 10 and 12), which was severe in 2. Neutrophil levels normalised after infection resolved and with IRT, and we did not observe any recurrences.
The distinctive feature of patients with XLA is a marked reduction in all classes of Ig and B cell types. In our study, all patients had B cell counts below 2%, and most had extremely low levels (<1%). We also observed low levels of all classes of Igs, with 78% of the patients having IgG levels below 200mg/dL. We would like to highlight the difficulty of diagnosing patient 4 due to his atypical presentation of XLA, with recurrent otitis media and pneumonia but with serum IgG levels at around 720mg/dL. Further investigation revealed the absence of isohaemagglutinins, a poor response to vaccination, and very low B cell counts (0.6%), leading to the diagnosis of XLA, which was later confirmed by the identification of a BTK mutation.
Our data showed that a BTK mutation was found in all of our patients. According to various published studies, a positive family history is only found in 30–50% of XLA patients.1 In our series, 2 patients had a family history of XLA, but all the mothers were carriers. We found the same mutation in 2 cases, patients 3 and 4, who were siblings.
IRT is the cornerstone of treatment of XLA. There are 2 IRT preparations, IVIg and SCIg, both of which are available in Portugal. Data from observational studies show that IRT reduces the rates of infection and hospitalisation in patients with XLA.1,18 In our study, every patient had a favourable outcome with IRT: a low number of infections and no severe infections. All patients switched to SCIg for self-administration at the home to reduce the number of visits to the hospital. Another key feature in the ongoing management of these patients is the intensive antibiotic treatment of any suspected or confirmed infections.2 Patients also benefit from advice on how to prevent exposure to pathogens.
It has been observed that respiratory infections are a considerable clinical problem even when IRT is underway.1,4 Some studies have shown that pneumonia and chronic or acute sinusitis are the most common respiratory tract infections after initiation of IRT,2,3 infections that were also found in our patients: most of them had mild infectious complications, which were most frequently otitis medias (67%). Our data did not show any severe infections during IRT.
There are known limitations to IRT. Treatment with high-dose IRT cannot be expected to contribute significantly to the prevention of infectious sinusitis, not only because of the scarce availability of IgG at mucosal surfaces, but also because mucosal protection is mostly provided by secretory IgA and IgM antibodies, which are also lacking in XLA.13 The finding of a significantly reduced incidence of severe bacterial infections with residual concentrations of serum IgG above 800mg/dL compared to 500mg/dL suggests that the 500mg/dL threshold is too low.10 Our patients received replacement doses aimed at reaching a threshold of 800mg/dL, and all but one achieved stable high concentrations (Fig. 1); one boy that had severe proteinuria (patient 5) was not able to maintain high levels in spite of receiving high-dose IRT.
Several studies show that even if patients with XLA are treated correctly, they may end up developing a chronic respiratory disease,4 underscoring the importance of monitoring patients in order to detect any subclinical but progressive lung damage.3,4 This followup is performed by means of pulmonary function tests and chest radiology.4 An Italian study of 73 patients found no association between the development of chronic lung damage and the age at diagnosis or the serum Ig levels.13 Two patients (22%) developed bronchiectases during the follow-up period, 3 and 11 years after initiation of IRT. They had pneumonia at the time of diagnosis, after which computer tomography of the lungs was normal, and received adequate IgG replacement therapy. This poses the problem that adequate serum IgG levels were not enough to prevent this complication, and that there must be other factors at play in the development of bronchiectases, such as low mucosal levels of IgA and IgG.
Recurrent conjunctivitis was an important and hard to manage problem in our patients (44%). Some experts recommend the topical administration of IgG, but its use is not based on evidence and there are no ocular preparations.
The data on the risk of cancer in patients with XLA is contradictory, especially as it pertains to lymphoid malignancies.19 The relationship between XLA and cancer has yet to be determined.19 The data in our study did not include any cancer cases.
The prognosis of XLA has improved considerably in the past 25 years thanks to earlier diagnoses, the use of IRT to raise levels above established thresholds, and early treatment of infections. Good outcomes can be achieved with an early diagnosis and immediate initiation of IRT, as observed in our series. The number of patients included in the study was small, so we could not reach any other conclusions. Gaining a better understanding of the pathophysiology of mucosal damage (ocular, respiratory and gastrointestinal) would be helpful in order to develop better therapeutic strategies and prevent complications.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fernandes A, Guedes M, Vasconcelos J, Neves E, Fernandes S, Marques L. Agammaglobulinemia ligada al cromosoma X: experiencia en un hospital portugués. An Pediatr (Barc). 2015;82:166–171.
Previous presentation: This study was presented as an oral communication at the XXXVIII Reunião Anual da Sociedade Portuguesa de Imunologia—Dos distúrbios imunológicos às imunoterapias, November 25–27, 2012, Porto, Portugal.