Our intent in writing this article is mainly practical, as we aim to discuss the optimal timing for discontinuation of treatment with gonadotropins in infants with hypogonadism through the presentation of 2 clinical cases.
In humans, the development and activity of the hypothalamic-pituitary-gonadal axis starts in the second trimester of gestation (mainly with a role in differentiation) and continues through the end of fertile life.
Hypogonadism (especially hypogonadotropic hypogonadism) is an ideal model to analyse the integrated regulation of hormones in the hypothalamic-pituitary-gonadal axis (especially as regards testicular development in infants).1
During infancy, in a stage known as “minipuberty”,2 there is an elevation in the levels of sex steroids and gonadotropins similar to the one experienced during puberty, although of lesser intensity. This period of “axis activation” lasts approximately 6 months in boys and 2–3 years in girls. In males, this is a critical period not only for the development of external genitalia, but also for the differentiation of Sertoli cells and therefore for future fertility.
Since female patients do not present any phenotypic abnormalities, diagnosis at this age is difficult unless they exhibit panhypopituitarism. In any case, at this age treatment is only given to male patients.
Treatment with gonadotropins is the most natural treatment that can be used in the first year of life. The first case of treatment in a male was reported in 2002,3 with initiation of treatment at age 8 months and a good growth response in the testicle, but not in the penis, which required further treatment with testosterone at a later age.
Administration of recombinant human follicle-stimulating hormone (FSH) and human chorionic gonadotropin (HCG) in the first 6–7 months of life stimulates Sertoli cell function (the effect can be assessed by measuring the levels of inhibin B and anti-Müllerian hormone [AMH]) and Leydig cell function while these cells exist (assessing the effect by measuring testosterone or insulin-like 3 [INSL3] protein levels).
In many cases, the micropenis in these infants is associated with cryptorchidism and small testicular volume. Treatment with gonadotropins at this age can increase intratesticular gonadotropin concentrations without risk of inducing spermatogenesis or a negative impact in the number of Sertoli cells, as these do not express androgen receptors until age 5 years. Treatment with FSH can increase testicular volume (as well as AMH and inhibin B levels) and treatment with HCG can increase levels of testosterone and INSL3.
However, there is still little experience with this pharmacologic approach, and there are no established protocols for its dosage or durations.
Treatment must be initiated as early as possible (thus the importance of early diagnosis), ideally within 6 months from birth. Although the literature on dosage is scarce, it has been proposed that treatment be initiated with a combination of FSH (37.5–75IU) administered subcutaneously 2 or 3 times a week and HCG (250–500 UI) administered subcutaneously twice a week.4
Treatment is maintained until at least age 6–7 months, the maximum clinical effectiveness is achieved (normal testicular volume and penile length) or testosterone levels decrease (which would signal a decrease in the population of Leydig cells).5,6
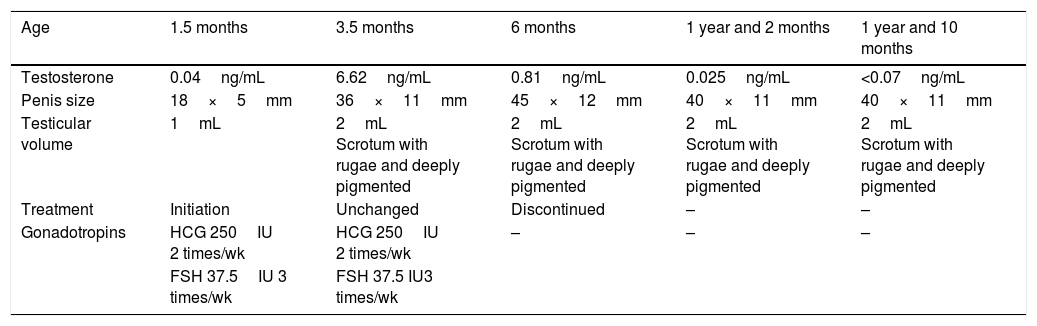
We now describe the first clinical case, corresponding to a newborn that presented with hypoglycaemia and micropenis and received a diagnosis of hypogonadotropic hypogonadism. Treatment with gonadotropins (Table 1) was initiated at age 1 month and a half and maintained until 6 months, when there was a marked decrease in testosterone levels despite the continued administration of gonadotropins. This decrease marks the end of “minipuberty” and thus the ideal time to end treatment.
Evolution and outcome of case 1.
| Age | 1.5 months | 3.5 months | 6 months | 1 year and 2 months | 1 year and 10 months |
|---|---|---|---|---|---|
| Testosterone | 0.04ng/mL | 6.62ng/mL | 0.81ng/mL | 0.025ng/mL | <0.07ng/mL |
| Penis size | 18×5mm | 36×11mm | 45×12mm | 40×11mm | 40×11mm |
| Testicular volume | 1mL | 2mL Scrotum with rugae and deeply pigmented | 2mL Scrotum with rugae and deeply pigmented | 2mL Scrotum with rugae and deeply pigmented | 2mL Scrotum with rugae and deeply pigmented |
| Treatment | Initiation | Unchanged | Discontinued | – | – |
| Gonadotropins | HCG 250IU 2 times/wk | HCG 250IU 2 times/wk | – | – | – |
| FSH 37.5IU 3 times/wk | FSH 37.5 IU3 times/wk |
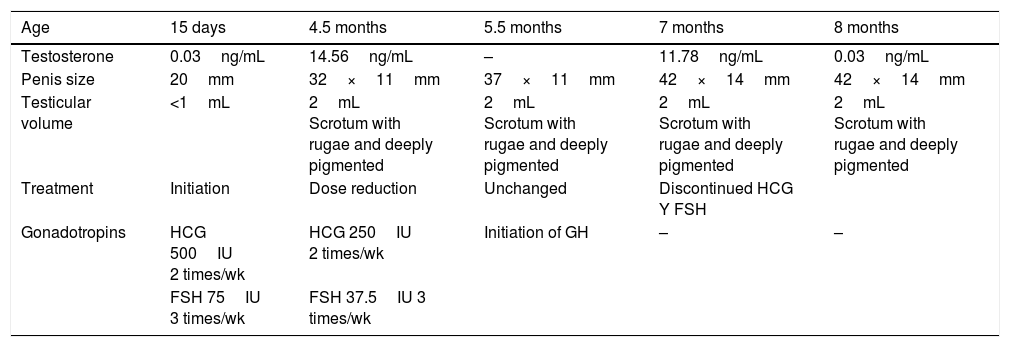
The second case corresponds to another male patient that also received a diagnosis of hypogonadotropic hypogonadism that started treatment with HCG and FSH at age 15 days. We monitored treatment by measuring the levels of testosterone, which exhibited a sharp elevation at age 5 months, upon which the dose given was halved. There was another sudden drop in testosterone at age 8 months, which led to discontinuation of treatment (Table 2).
Evolution and outcome of case 2.
| Age | 15 days | 4.5 months | 5.5 months | 7 months | 8 months |
|---|---|---|---|---|---|
| Testosterone | 0.03ng/mL | 14.56ng/mL | – | 11.78ng/mL | 0.03ng/mL |
| Penis size | 20mm | 32×11mm | 37×11mm | 42×14mm | 42×14mm |
| Testicular volume | <1mL | 2mL Scrotum with rugae and deeply pigmented | 2mL Scrotum with rugae and deeply pigmented | 2mL Scrotum with rugae and deeply pigmented | 2mL Scrotum with rugae and deeply pigmented |
| Treatment | Initiation | Dose reduction | Unchanged | Discontinued HCG Y FSH | |
| Gonadotropins | HCG 500IU 2 times/wk | HCG 250IU 2 times/wk | Initiation of GH | – | – |
| FSH 75IU 3 times/wk | FSH 37.5IU 3 times/wk |
To conclude, while further studies are required, it is reasonable to assert that treatment of hypogonadotropic hypogonadism should start as early as possible with the aim of simulating the “minipuberty” stage. As for its monitoring, in addition to observing the growth of the penis and testes, measurement of testosterone levels is useful, as they can provide an accurate marker of the end of minipuberty and therefore of the right time to discontinue treatment.
Please cite this article as: Álvarez Casaño M, López Siguero JP. Monitorización del tratamiento del hipogonadismo hipogonadotropo en el lactante. An Pediatr (Barc). 2019;90:190–192.