Non-IgE-mediated cow's milk allergy is a frequent disorder in paediatrics. As patients might be seen by professionals from different specialties and levels of expertise, a great variability in diagnostic procedures and disease monitoring is commonly observed. Therefore, four scientific societies involved in its management have developed a consensus document providing specific recommendations related to its prevention, diagnosis, treatment and follow up.
La alergia a las proteínas de leche de vaca no mediada por IgE es una patología frecuente, en cuyo manejo están implicados profesionales de diferentes áreas existiendo a día de hoy una gran variabilidad en la forma de abordar su diagnóstico, tratamiento, seguimiento y prevención. Con el objetivo de unificar pautas de actuación se ha elaborado un documento de consenso entre cuatro de las sociedades científicas implicadas en el abordaje de niños con dicha patología.
Cow's milk protein allergy (CMPA) is the most frequent food allergy in infants aged less than 1 year. It results from a maladaptive immune response (IgE-mediated, non-IgE-mediated or mixed) against cow's milk proteins (CMPs).1,2 Despite the availability of different guidelines and recommendations for the management of children with CMPA,2–11 there is evidence of a wide variability in its diagnosis and treatment in Spain, especially in cases of non-IgE-mediated allergy.12 This document presents the recommendations of a multidisciplinary group of paediatric experts based on the current evidence with the purpose of standardising the approach to the diagnosis, treatment, followup and prevention of CMPA not mediated by IgE (non-IgE CMPA) in children aged less than 2 years in Spain, at both the primary care and the specialty care levels. This document does not address the management of IgE-mediated CMPA, atopic dermatitis, eosinophilic oesophagitis, gastritis or gastroenteritis or adverse reactions that are not caused by an immune mechanism.
Method for the development of the consensus document- •
Working group. It comprised 11 experts representing 4 paediatric societies: Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (Spanish Society of Paediatric Gastroenterology, Hepatology and Nutrition, SEGHNP), Asociación Española de Pediatría de Atención Primaria (Spanish Association of Primary Care Paediatrics, AEPAP), Sociedad Española de Pediatría Extrahospitalaria y Atención Primaria (Spanish Society of Outpatient and Primary Care Paediatrics, SEPEAP) and Sociedad Española de Inmunología Clínica, Alergología y Asma Pediátrica (Spanish Society of Paediatric Clinical Immunology, Allergology and Asthma, SEICAP). Once the group agreed on the aspects that needed to be considered in the areas of clinical manifestations, diagnosis, treatment, followup and prevention of non-IgE CMPA, the list was distributed for review based on personal experience.
- •
Literature search. We conducted 2 searches in PubMed/MEDLINE (Appendix B, online supplemental material, Figure S1), submitting the resulting list of articles to all the authors, who each selected the articles that were relevant for addressing specific questions.
- •
Drafting of the document. After reviewing the selected articles, the authors expressed their results as statements, basic concepts and recommendations that were later put to a vote by the whole group. The entire document can be consulted in the webpages of each of the societies that participated in its development.
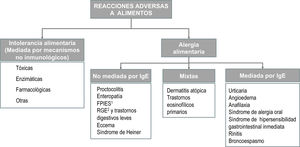
The term allergy refers exclusively to adverse reactions involving one or several immune mechanisms—proven or highly suspected—and must be distinguished from reactions due to enzymatic, toxic or pharmacological mechanisms3,13 (Appendix B, online supplemental material, Figure S2). From a clinical standpoint, reactions mediated by IgE are characterised by the acute onset of a predominantly cutaneous or respiratory response associated to the presence of specific IgE antibodies. Reactions that are not mediated by IgE usually result from cellular immune responses, although in most cases the involvement of an immune mechanism cannot be proven.
The only tools available for diagnosis of non-IgE CMPA are a detailed history and elimination of CMP from the diet followed by an oral challenge.14–16 The first lays the foundation to suspect the presence of allergy, while the second is necessary to confirm the diagnosis.
The delayed-onset symptoms are predominantly gastrointestinal, and they may present as any of these three syndromes: food protein-induced allergic proctocolitis, food protein-induced enteropathy and food protein induced enterocolitis syndrome (FPIES)17 (Appendix B, online supplemental material, Table S1). International consensus guidelines for the management of the latter have been published recently9 (Table 1). There are no specific diagnostic criteria for the other two, which must be diagnosed based on the clinical presentation (Table 2). Furthermore, non-IgE CMPA may mimic gastrointestinal disorders that are frequent in this age group, such as gastro-oesophageal reflux (GOR), infant colic and constipation.17–21 The presence of a family history of atopy, involvement of several systems (gastrointestinal, cutaneous, respiratory manifestations) and absence of improvement with customary treatment suggests the presence of non-IgE CMPA in these patients.15,16,20,21
Recommendation 1. A detailed clinical history—including a physical examination, growth assessment and feeding history—is the key to establish diagnosis suspicion.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 2. A diagnosis of non-IgE CMPA should be considered in:
2a: infants presenting with one or more of the following symptoms: prolonged diarrhoea, poor growth, recurrent vomiting, abdominal distension, blood in stools, iron-deficiency anaemia or other persistent, mild gastrointestinal manifestations that do not respond to customary treatment. The concomitant presence of cutaneous or respiratory manifestations suggestive of atopy intensifies the clinical suspicion.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
2b: patients presenting with the features that characterise the three gastrointestinal syndromes mentioned above: allergic proctocolitis, enteropathy or FPIES.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 3. In children with GOR, persistent constipation or colic that do not respond to customary management, improvement or resolution of symptoms after the elimination of CMP from the diet supports the suspected diagnosis of non-IgE CMPA, and requires performance of an oral food challenge for confirmation.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Diagnostic criteria for FPIES due to CMP.
| Acute FPIES due to CMP: |
| Requires the presence of the major criterion and at least 3 minor criteria associated with ingestion of CMP |
| Major criterion: |
| Vomiting within 1 to 4h from ingestion of CMP and absence of classic IgE-mediated allergic skin or respiratory symptoms |
| Minor criteria: |
| 1. A second (or more) episode of repetitive vomiting after eating CMP |
| 2. Repetitive vomiting episode 1–4h after eating a different food |
| 3. Lethargy |
| 4. Marked pallor |
| 5. Need for emergency department visit to control the adverse reaction |
| 6. Need for intravenous fluid support to control the adverse reaction |
| 7. Diarrhoea within 24h (usually in 5–10h) of ingestion of CMP |
| 8. Hypotension |
| 9. Hypothermia (body temperature ≤35°C) |
| Chronic FPIES due to CMP: |
| Requires development of symptoms meeting the criteria for diagnosis of the acute form following an oral challenge |
| • Severe presentation (when CMP is ingested on a regular basis): intermittent but progressive vomiting and diarrhoea (occasionally with blood), sometimes with dehydration and metabolic acidosis |
| • Milder presentation (when lesser amounts of CMP are consumed): intermittent vomiting and/or diarrhoea, usually with poor weight gain/failure to thrive but without dehydration or metabolic acidosis |
Adapted from Nowak-Wegrzyn et al.9
Clinical characteristics of enteropathy and proctocolitis.
| Cow's milk protein-induced proctocolitis: |
| Diagnosis requires performance of an oral challenge 2–4 weeks after elimination of CMP, as long as the symptoms have disappeared |
| • Presence of fresh, red blood in the stools of an otherwise healthy infant fed human milk (from mother whose diet includes dairy) or formula containing CMP |
| • Absence of faltering growth |
| • Absence of general malaise |
| • Negative stool cultures |
| • Resolution of bleeding in the 4 weeks following exclusion of CMP from the diet (or exclusion from mother's diet in case of breastfed infants) |
| • Recurrence of symptoms following food challenge |
| Cow's milk protein-induced enteropathy: |
| Diagnosis requires performance of an oral challenge 4–6 weeks after elimination of CMP, once the symptoms have disappeared |
| • Anorexia and refusal to feed |
| • Initially, the patient may develop intermittent vomiting and constipation |
| • Diarrhoea of more than 15 days’ duration with or without faltering growth that resolves in the 4 weeks following elimination CMP from the diet of the child |
| • Diarrhoea reappears with an insidious and progressive course after reintroducing CMP in the diet |
When CMPA is suspected in a young child based on the clinical history, CMP should be eliminated from the diet. Elimination leads to improvement and resolution of symptoms in a variable period of time, which may be of 1 to 5 days in acute forms (acute FPIES, vomiting), 1–2 weeks in cases of eczema or rectal bleeding, and up to 2–4 weeks in patients with constipation, diarrhoea and/or growth faltering.22 The CMP-free diet is to be maintained until symptoms resolve fully, and should not be prolonged past 6 weeks without confirmation of the diagnosis by means of an oral challenge. Not performing the challenge should only be considered in patients in whom repeated exposure is deemed too risky due to the severity of the initial reaction.
Recommendation 4. The group of experts does not recommend the following for confirmation of the diagnosis:
4.a: performance of a prick test and/or testing for specific IgE against CMP, unless there are doubts concerning the involvement of an IgE mechanism.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
4.b: routine performance of laboratory tests.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
4.c: performance of the patch test.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
4.d: routine performance of endoscopy, unless the diagnosis is uncertain or the patient does not respond to exclusion of CMP from the diet, with endoscopy being performed based on the judgement of the gastroenterologist.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 5: the diagnosis of non-IgE-mediated CMPA requires elimination of CMP from the diet for a period not exceeding 6 weeks (trial of elimination), verification of the resolution of symptoms, and controlled reintroduction of CMP (oral food challenge), except in cases of severe FPIES.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
In cases manifesting with proctocolitis, disorders such as GOR, constipation or colic, the symptoms resulting from reintroduction of CMP are usually mild and easily managed at the outpatient level, so the challenge can be performed at home under the supervision of a paediatrician (Table 3). If there is suspicion of an IgE mechanism (onset of symptoms within 2 hours of ingestion and/or development of cutaneous and respiratory symptoms associated with IgE-mediated reactions), severe atopic dermatitis, or moderate-to severe FPIES or enteropathy, reintroduction of CMP may involve a considerable risk, and should therefore be performed in the hospital (Appendix B, Table 4 and online supplemental material, Table S2) (Fig. 1).
Oral food challenge at home.
| Requirements |
| - Complete resolution of clinical manifestations with the CMP-free diet |
| - In case of intercurrent disease, especially infectious or respiratory, the challenge should be postponed until symptoms resolve and at least 1 week has elapsed since completion of the treatment administered to control the disease |
| The oral challenge should not be performed at home in case of: severe clinical presentation, FPIES, clinical suspicion of an IgE-mediate mechanism, positive result of specific IgE or prick test for CMP |
| Method |
| • In formula-fed children: substitute infant formula* for one measure of special formula in at least 2 of the feeds. If the child does not exhibit symptoms, once the switch is complete in those 2 feeds, one bottle of special formula may be replaced by one of infant formula* each day until the reintroduction is complete |
| • In breastfed children: reintroduce cow's milk and dairy products in mother's diet (start with 1 serving of milk or dairy products the first week and, should the child not exhibit symptoms, progressively increase the amount of dairy in the diet) |
| Monitor for the potential development of symptoms for 4 weeks after reintroduction of dairy |
| If symptoms suggestive of CMPA develop during the challenge, administration of CMP should be discontinued immediately |
| New foods should not be introduced in the diet while the oral challenge is underway |
Oral food challenge in hospital.
| Protocol |
| 1. Measure patient's current weight |
| 2. Ensure that a specific IgE test and/or prick test for CMP have been performed recently with negative results* |
| 3. Establish venous access** |
| 4. Calculate the amount of formula required in g of protein and administer orally divided in 3 doses at 30–45min intervals*** |
| The total dose will be of 0.06–0.6g per kg of body weight (usually 0.15–0.3g per kg of body weight), not to exceed 10g |
| 5. Observe the patient for 4h after the last dose |
| 6. If the patient exhibits tolerance, continue challenge at home with consumption of 150–200mL of cow's milk or formula for 2 weeks. Thereon, if the patient does not exhibit any associated adverse events, progressively remove restrictions from diet |
Adapted from Nowak-Wegrzyn et al.9
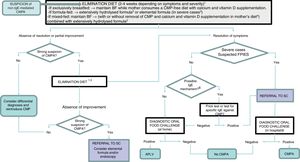
Diagnostic algorithm.
BF, breastfeeding; CMP, cow's milk protein; CMPA, cow's milk protein allergy; FPIES, food protein-induced enterocolitis syndrome; SC, specialty care.
1 Improvement with the elimination diet can be expected after a variable period of time depending on the clinical presentation: between 1 and 5 days in acute forms (acute FPIES, vomiting); 1–2 weeks in cases of eczema and gastrointestinal bleeding; 2–4 weeks in cases of constipation, diarrhoea and growth faltering.
2 Eliminate lactose as well in case of suspected lactose intolerance, enteropathy and/or growth faltering.
3 Patients with significant growth faltering (malnutrition, hypoalbuminaemia), rectal bleeding associated with haemodynamic instability or severe FPIES.
4 When symptoms develop with the first feeds of formula in infants that were previously breastfed and asymptomatic, BF should be recommended and elimination of CMP from the mother's diet is not necessary.
5 Switch to another extensively hydrolysed formula of different characteristics or a hydrolysed rice formula. Elimination of soy and egg (in the child and/or mother) if a concomitant food allergy is suspected.
6 An IgE-mediated mechanism should be suspected in case of immediate reactions (onset less than 2h after intake) and/or development of cutaneous and respiratory manifestations associated with IgE mechanisms (urticaria, erythema, oedema, bronchospasm).
7 In case it is not available at the primary care level, refer patient to specialty care.
8 The oral food challenge can be foregone in severe cases and cases meeting the diagnostic criteria for FPIES.
In patients with severe atopic dermatitis and/or FPIES, performance of a prick test or specific IgE tests is recommended before starting the challenge for diagnosis. In case the result of either test is positive, the oral challenge will be performed according to the protocol for IgE-mediated reactions.
The oral challenge is interpreted based on clinical features, that is, the resurgence of symptoms, although these can take 1 to 2 weeks to develop (or 2–4 weeks in cases manifesting with altered intestinal function or eczema) or not be sufficiently pronounced in the initial days when the intake of CMP is lower. For this reason, observation in uncertain cases should be maintained for at least 4 weeks after reintroducing CMP in the diet. The Cow's Milk-related Symptom Score may be helpful in the evaluation of mild forms with symptoms similar to those of functional disorders.20,21
Recommendation 6. In patients with proctocolitis, GOR, colic, constipation and other mild gastrointestinal symptoms, the oral challenge may be performed at home under the supervision of the paediatrician.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 7. In cases with immediate reactions, severe atopic dermatitis, FPIES, moderate to severe enteropathy or in whom an IgE-mediated mechanism is suspected, the oral challenge will always be performed in hospital.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 8. In the challenge, the period of observation after reintroduction of CMP in the diet, should symptoms not develop readily, should be of at least 2 weeks and of up to 4 weeks, especially in cases presenting with constipation or enteropathy.
Vote: 10 agreed, 1 abstained, 0 disagreed. 91% consensus.
The treatment of non-IgE-mediated CMP consists in the elimination of CMP from the diet. In exclusively-breasted infants, maintenance of breastfeeding must be prioritised, having the mother follow a CMP-free diet. Persistence of symptoms despite elimination from the maternal diet may be due to sensitisation to other foods (mainly soy and egg), whose exclusion should be considered, too. If the onset of symptoms is associated with the initiation of complementary feeding with formula or dairy products, exclusion of CMP from the mother's diet is not considered necessary.23,24
Recommendation 9. In exclusively-breastfed children, breastfeeding must continue, always recommending exclusion of CMPs from the mother's diet. If the symptoms persist despite adequate adherence to the CMP-free diet, it is recommended that breastfeeding is maintained, considering the exclusion of other potentially involved foods (especially soy and/or egg).
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 10. In infants receiving mixed breastfeeding, if the onset of symptoms coincides with the introduction of formula feeds, breastfeeding should be maintained, and in most cases elimination of CMP from the mother's diet is not necessary.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Different formulas approved for treatment of infants with CMPA are currently available. Among these, extensively hydrolysed formulas (EHFs) based on casein and/or whey CMP are considered the first choice.2–11,25,26 Administration of EHFs enriched with medium chain triglycerides should be considered in infants with growth faltering, including formulas containing lactose if lactose intolerance is not suspected. Although several studies published in recent years found a positive impact of formulas supplemented with probiotics in the development of tolerance,27–29 further research is required to confirm this association.
In children that refuse or cannot tolerate EHFs or families following a vegan diet, hydrolysed rice protein formulas have been proven efficacious and safe.2–11,30 The use of soy-based formulas is not recommended in infants aged less than 6 months.2 In severe cases with significant growth faltering or with rectal bleeding associated with haemodynamic instability, elemental formulas based on free amino acids are the first line of treatment.31
Recommendation 11. In children fed with infant formula that develop non-IgE-mediated CMPA:
11.a: EHF of casein or whey CMP constitute the first-line treatment of mild to moderate forms.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
11.b: Hydrolysed rice protein formulas may be prescribed at any age and can offer an alternative to patients that refuse or do not respond to treatment with casein or whey CMP EHF. We recommend against the use of soy-based formulas in infants aged less than 6 months.
Vote: 10 agreed, 1 abstained, 0 disagreed. 91% consensus.
11.c: Amino acid-based formulas (elemental formulas) constitute the first-line treatment in severe cases of enteropathy or FPIES. They can also be used as an alternative in patients that do not respond to treatment with casein or whey-CMP EHFs.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 12. There is not sufficient evidence to recommend the routine use of formulas enriched with prebiotic and/or probiotics in the management of children with CMPA.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
There is a high probability that hydrolysed formulas and milks or formulas based on the milk of other mammals (sheep, goat, buffalo) will not be tolerated by children with CMPA.2–11 Plant-based drinks made with soy, rice, oat, quinoa, tiger nut or almond are of little nutritional value and have low protein and energy contents, unlike plant-based infant formulas. These plant-based milks should never be given as a replacement for cow's milk, although they may be given as part of a varied diet to children aged more than 2 years.2,6,31
Recommendation 13. Partially hydrolysed formulas, formulas or milks from other mammals (goat, sheep, buffalo, mare, camel, donkey) and plant-based milks (soy, rice, oat, almond, tiger nut etc.) should not be used in the management of children with CMPA.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
We must remember that mothers and infants with a CMP-free diet are at risk of dietary vitamin D and calcium deficiency. It is recommended that mothers receive supplementation for both. Infants will be given vitamin D, and calcium supplementation will be considered in case of insufficient dietary intake.15,32,33
Recommendation 14. A CMP-free diet must be prescribed after diagnosis. Products labelled as possibly containing “traces” of CMP need not be eliminated if the child can tolerate them.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 15. Medical supervision of restrictive diets is always recommended, preferably in collaboration with a nutritionist/dietitian.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 16. Mothers that need to eliminate CMP from their diet for the management of CMPA in their children should receive supplementation with calcium (1g/day) and vitamin D (600IU/day).
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 17. In infants and children who are not breastfed, we recommend ingestion of a suitable amount of formula guaranteeing, among others, an adequate intake of calcium. If there is evidence that intake is insufficient, supplementation with calcium is recommended. In addition, supplementation with vitamin D (400IU/day) should be maintained for the duration of the restricted diet.
Vote: 10 agreed, 1 abstained, 0 disagreed. 91% consensus.
Most children with CMPA tolerate ingestion of well-cooked beef and other bovine meat (ox, bull, etc.). Thus, unless the child exhibits clinical manifestations associated to their consumption, these meats need not be avoided.10
Recommendation 18. Complementary feeding in children with CMPA should adhere to the guidelines applied to any other child under similar circumstances, save for the exclusion of CMP from the diet.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 19. In general, beef and similar meats, always well cooked, can be included in the diet of children with CMPA.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
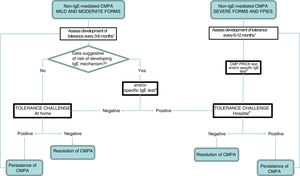
The persistence or resolution of CMPA can only be established by testing for the development of tolerance, which involves the gradual reintroduction of CMP under medical supervision. Tolerance should be evaluated at regular intervals according to the clinical features and the severity of the response in previous challenges. Patients with mild forms manifesting with GOR, colic, constipation and proctocolitis may develop tolerance early, at ages 3–6 months, while patients with FPIES develop it later, and the oral challenge for tolerance testing should be deferred to ages 12, 18 or even 24 months in patients with the most severe forms.9,17
Recommendation 20. When it comes to followup, the group of experts does not recommend:
20.a: prescription of self-injectable epinephrine to children with FPIES except in case of a concomitant IgE-mediated allergy.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
20.b: routine performance of diagnostic tests.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
20.c: changes to the vaccination schedule.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 21. Treatment with a CMP-free diet should be maintained for a variable period: from 3 to 6 months in mild forms, to up to 12 months in the most severe cases. In case of unfavourable response to reintroduction of CMP, tolerance must be re-evaluated periodically every 6 to 12 months under medical supervision.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Before reintroducing CMP in the diet, the clinician should consider whether testing for specific IgE and/or a prick test are necessary. In so-called atypical FPIES, these tests may be positive at diagnosis or at a later time, and patients tend to develop tolerance later.9,34,35 Although most maintain a clinical presentation characteristic of non-IgE-mediated allergy, approximately 35% of patients also exhibit manifestations characteristic of IgE-mediated allergy.34 Furthermore, patients that have exhibited severe reactions, immediate reactions (onset within 2 hours from intake) or have a personal history of atopy (atopic dermatitis, recurrent bronchospasm, allergic rhinitis and/or IgE-mediated hypersensitivity to other foods) should be considered at high risk of developing IgE-mediated reactions after a prolonged period of elimination6 (Fig. 2).
Followup algorithm.
CMP, cow's milk protein; CMPA, cow's milk protein allergy; FPIES, food protein-induced enterocolitis syndrome.
1 The intervals between challenges for evaluation of tolerance will be determined by the clinician, and we recommend a higher frequency the milder the presentation. In case of dietary transgressions associated with symptoms, postpone challenge.
2 The patients considered at risk of developing an IgE-mediated response include those with a personal history of atopy and those that developed symptoms immediately after ingestion of CMP.
3 If not available at the primary care level, refer to specialty care.
4 Perform according to IgE-mediated protocol in case of positive prick/IgE test.
The development of tolerance can be assessed at home with the gradual and controlled reintroduction of CMP in patients with non-IgE CMPA that have mild or moderate symptoms6,7,15 (Fig. 3), and should be assessed in a hospital setting if there is risk of a systemic reaction (FPIES or severe enteropathy) or an IgE-mediated reaction (children that have had positive results of testing for specific IgE and/or the prick test during the followup)9 (Table 4).
Recommendation 22. In patients with a personal history of atopy, immediate reactions (onset within 2 hours from ingestion), FPIES and all severe forms of allergy, a specific IgE test and/or a prick test will be performed before reintroducing CMP to assess tolerance.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Recommendation 23. In cases of severe allergy, FPIES or evidence of sensitisation to CMP based on the results of specific IgE or prick tests, tolerance should always be tested in a hospital setting. In mild cases, testing for tolerance can be performed at home under medical supervision.
Vote: 11 agreed, 0 abstained, 0 disagreed. 100% consensus.
Milk ladder challenge to assess tolerance at home. Adjust fed products to the patient's age.
BF, breastfeeding; CM, cow's milk.
1 Simple biscuits, such as María biscuits (no chocolate, custard, etc.). To feed infants, they can be puréed with fruit.
2 Adding fresh fruit or sweetening lightly with some milk of sugar is acceptable.
3 In children that continue to breastfeed, introduction of cow's milk will be delayed until breastfeeding is discontinued or supplementation becomes necessary.
4 Extensively hydrolysed formula, hydrolysed rice protein formula or soy-based formula.
5 Eventually, introduce a lactose-free formula, and later switch to an adapted formula.
6 In children that are not fed special formulas (for instance, fed plant-based milks) CMP could be reintroduced with low-fat milk (to facilitate acceptance), switching to whole milk 2 weeks later.
The data on potential risk factors for non-IgE CMPA are scarce, as most studies have been conducted in patients with IgE-mediated allergy.36–38 At present, no intervention for preventing the development of non-IgE CMPA is backed by sufficient evidence.39–43 Exclusive breastfeeding is the optimal source of nutrition for infants up to age 6 months, and while the evidence on its protective effect against allergy development is inconsistent, it should still be recommended on account of the numerous benefits it offers.43
Conflicts of interestBeatriz Espín Jaime has participated in educational activities and symposiums sponsored by different infant and child food manufacturers: Mead Johnson, Hero, Nestlé, Nutricia, Ordesa, Nutribén, Lactalis. She has been a consultant in Nutricia advisory boards.
Juan J. Díaz Martín has received fees for participating in talks and symposia organised by different infant and child food manufacturers: Mead Johnson, Hero, Nestlé, Nutricia, Ordesa, Nutribén. He has received grants to attend scientific congresses from several corporations (Mead Johnson, Nestlé, Ordesa, Nutricia) and has been a consultant on Nutricia and Abbvie advisory boards.
Luis Carlos Blesa Baviera has received fees or support of various types (room and board, registration fees, travel) from most corporations related to infant and child foods for speaking, participating and/or attending a variety of workshops, congresses, conferences and meetings related to paediatrics.
Anselmo Hernández Hernández has participated in round tables and conferences sponsored by, and attended workshops and congresses for which he received financial support (for registration, travel and room and board) from infant and child food manufacturers and other corporations.
Cristóbal Coronel Rodríguez has participated as a facilitator or speaker in round tables, workshops and symposiums sponsored by infant and child food manufacturers.
Enriqueta Román Riechmann has participated in educational activities funded by Alter, Casen Fleet, Ferrer Internacional, Ferring, Hero, Mead Johnson and Nestlé.
Carmen Ribes Koninckx has received fees for participating in talks and symposiums organised by different manufacturers of infant and child foods: Mead Johnson, Nestlé, Nutricia. She has acted as a consultant in Nutritia, Nestlé and Mead Johnson advisory.
Ángela Claver Monzón, José Ignacio García Burriel, María José García Mérida and Celia Pinto Fernández have no conflicts of interest to declare.
Please cite this article as: Espín Jaime B, Díaz Martín JJ, Blesa Baviera LC, Claver Monzón Á, Hernández Hernández A, García Burriel JI, et al. Alergia a las proteínas de leche de vaca no mediada por IgE: documento de consenso de la Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (SEGHNP), la Asociación Española de Pediatría de Atención Primaria (AEPAP), la Sociedad Española de Pediatría Extrahospitalaria y Atención Primaria (SEPEAP) y la Sociedad Española de Inmunología Clínica, Alergología y Asma Pediátrica (SEICAP). An Pediatr (Barc). 2019;90:193.
Previous presentation: This document was presented in a round table at the 66 Congress of the Asociación Española de Pediatría; June 7–9, 2018; Zaragoza, Spain.