Human parechovirus (HPeV) is one of the recently described picornaviridae viruses that have been associated with fever without source (FWS), clinical sepsis, gastroenteritis, meningitis, or encephalitis in very young infants. The aim of this study is to describe the epidemiology and clinical features of these viruses.
Patients and methodsA prospective multicentre 3-year study was conducted in 12 hospitals in Spain. Out of 850 specimens examined, 47 were positive (5.52%), with HPeV-3 being the most frequent (29 cases). Infections occurred throughout the year, but mainly in May and July, and a biennial distribution was observed. More than half (57%) were neonates, and only 2 children were older than 3 months. Fever was present in all children, with irritability in 45%, rash in 18.6%, and diarrhoea in 14%. The results of biochemical tests were all in normal range. The most common final diagnosis was FWS (61%), followed by clinical sepsis (29%). Up to 29% of infants were admitted to the intensive care unit, but only one patient had sequelae.
ResultsOut of 850 specimens examined, 47 were positive (5.52%) for HPeV, with HPeV-3 being the most frequent (29 cases). Infections occurred throughout the year, but mainly in May and July, and a biennial distribution was observed. More than half (57%) were neonates, and only 2 children were older than 3 months. Fever was present in all children, with irritability in 45%, rash in 18.6%, and diarrhoea in 14%. The results of biochemical tests were all in normal range. The most common final diagnosis was FWS (61%), followed by clinical sepsis (29%). Up to 29% of infants were admitted to the intensive care unit, but only one patient had sequelae.
ConclusionsHPeV circulates in our country, mainly during spring and summer, and affects young infants with a FWS and clinical sepsis. Molecular diagnostic techniques in all hospitals could help in improving the management of patients with these infections.
Los parechovirus humanos (HPeV) son virus de la familia Picornaviridae, recientemente descritos, a los que se atribuyen cuadros de fiebre sin foco (FSF), sepsis clínica, gastroenteritis, meningitis o encefalitis fundamentalmente en lactantes pequeños. Nuestro objetivo fue describir la epidemiología y las características clínicas de las infecciones por HPeV en nuestro medio.
Pacientes y métodosEstudio multicéntrico prospectivo, llevado a cabo en 12 hospitales a nivel nacional, entre 2013-2015, en niños<3 años con FSF, sepsis clínica o patología neurológica. Se realizó determinación de HPeV mediante RT-PCR en el Centro Nacional de Microbiología en suero, heces o líquido cefalorraquídeo.
ResultadosSe analizan 47 infecciones por HPeV de un total de 850 muestras (5,52%), siendo HPeV-3 el más frecuente (29 casos), con predominio en mayo y julio, con una distribución bienal. El 57% eran neonatos y solo 2>3 meses. Todos los pacientes presentaron fiebre, el 45% irritabilidad, el 18,6% exantema y el 14% diarrea. No se observa ninguna alteración específica en las pruebas bioquímicas. El diagnóstico final más frecuente fue FSF (61%) seguido de sepsis clínica (29%). Aunque un 29% de los niños precisaron ingreso en cuidados intensivos, solo un paciente presentó secuelas.
ConclusionesLos HPeV circulan en nuestro país, afectando fundamentalmente a lactantes < 2 meses y se asocian a FSF y sepsis clínica, con un predominio en primavera y verano. Sería de interés implementar las técnicas moleculares de diagnóstico en todos los hospitales para reconocer y manejar adecuadamente estas infecciones.
Human parechoviruses (HPeVs) are small RNA viruses, of which 16 different types are currently known (HPeV-1 through 16).1,2 Types 1 and 2 were first described in 1945 and initially classified as echovirus 22 and 23 within the Enterovirus genus. In 1999 they were reclassified into a different genus, Parechovirus, within the Picornaviridae family, based on their biological and genomic characteristics. Infections by HPeV-1 and 2 had been associated with mild gastrointestinal and respiratory disease. However, types 3–16, described in the past 10–12 years, seem to be able to produce neurologic and systemic infections of varying severity. The studies published in recent years attribute HPeVs, and especially type 3, a significant role in severe infections in young infants,3 although the epidemiology and pathogenesis of these viruses have yet to be clearly established. Along with human enteroviruses (EVs), HPeVs are causative agents of aseptic meningitis and febrile disease in childhood.4,5 However, few data have been published to date on the incidence and circulation of HPeV in Spain.6 Therefore, our aim was to describe the clinical characteristics of documented systemic and neurologic infections by HPeV in a nationwide multicentre study on enterovirus and parechovirus infections in children.
Patients and methodsWe conducted a prospective study with the participation of 12 Spanish hospitals between 2013 and 2015. The study was funded by a grant of the Instituto Carlos III in the area of Strategic Action in Health (AES, PI12-00904) and approved by the Ethics Committee of this institution. The parents or legal guardians signed the informed consent for participation.
We included children aged less than 3 years admitted to the paediatrics or neonatal units of participating hospitals who presented with symptoms compatible with fever without source (FWS), aseptic meningitis, encephalitis or meningoencephalitis, myocarditis or clinical sepsis. We defined FWS as an axillary temperature of more than 37.9°C whose aetiology was not identified after a thorough physical examination and laboratory workup (conducted according to the usual diagnostic protocols of each centre). Aseptic meningitis was diagnosed when pleocytosis was present—defined as more than 30cells/mm3 in newborns or more than 5cells/mm3 in children aged more than 1 month—and bacterial culture of the cerebrospinal fluid (CSF) was negative. Encephalitis was diagnosed in patients with an altered level of consciousness and additional manifestations of neurologic involvement, without necessarily requiring the presence of pleocytosis in CSF or abnormal findings on magnetic resonance imaging examination or electroencephalogram, although these diagnostic tests guided the diagnosis. Meningoencephalitis was diagnosed based on the presence of clinical manifestations of both meningitis and encephalitis. We defined clinical sepsis as the presence of clinical features and biomarkers indicative of a systemic inflammatory response in the absence of confirmation of bacterial infection by culture. We collected clinical and laboratory data for the patients in a form designed for this purpose.
Cerebrospinal fluid, throat swab, stool or blood specimens were collected for analysis. The samples were submitted to the microbiology laboratories of participating hospitals, where infection by neurotropic herpes viruses was ruled out (herpes simplex 1 and 2 and varicella-zoster virus). The presence of EV was also assessed using molecular methods, and samples that tested negative for EV were submitted to the Enterovirus Unit of the Centro Nacional de Microbiología (National Centre of Microbiology), where they were assessed for the presence of HPeV by means of a RT-PCR protocol designed specifically for its detection.7 Parechoviruses detected by this method were typed by analysis of the VP3/VP1 region by the RT-PCR method described in a previous publication,7 gene sequencing and phylogenetic analysis.
We performed the statistical analysis with the Statistical Package for the Social Sciences (SPSS) version 13.0. We have summarised the data for discrete variables as percentages and those for continuous variables as mean and standard deviation (SD). We compared clinical characteristics and laboratory variables by means of the Student t, Mann–Whitney U, χ2 and Fisher exact tests. We defined statistical significance as a p-value of less than 0.05 in any of the tests.
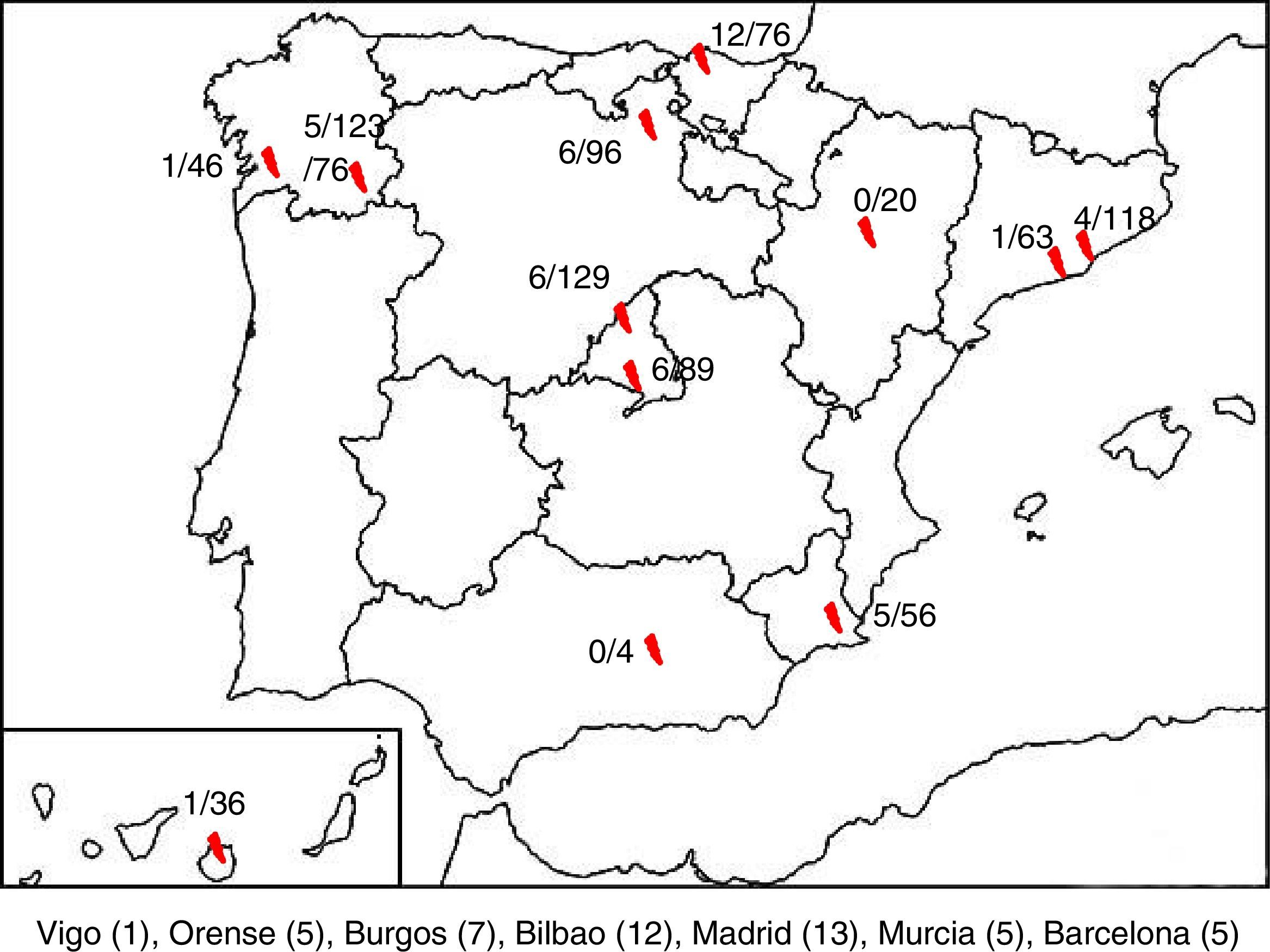
ResultsA total of 850 samples corresponding to 845 patients were collected, of which 47 tested positive to HPeV (5.52%). We were only able to obtain clinical data for 43 of these patients, so the other 4 were excluded from the analysis. There were HPeV-positive cases in 10 of the 12 hospitals. Fig. 1 shows the geographical distribution of these cases.
We analysed the epidemiological characteristics of the 43 cases of infection by HPeV, of which 24 occurred in boys and 19 in girls. Of all positive samples, 31 were CSF specimens (31/596 CSF specimens in the study; 5.2%), 11 blood specimens (11/145; 7.58%) and 1 a throat swab (1/91, 1.09%). Parechovirus was not detected in any of the specimens obtained from stools (13 samples), biopsy (4 samples) or bronchoalveolar lavage (1 sample). The median age was 24 days (interquartile range [IQR], 12–42 days), 26 of the cases were in newborns (age<28 days) and only 2 in children aged more than 3 months. Two cases were in children born preterm. All of the patients were at home when the onset of symptoms occurred. None were enrolled in a childcare facility.
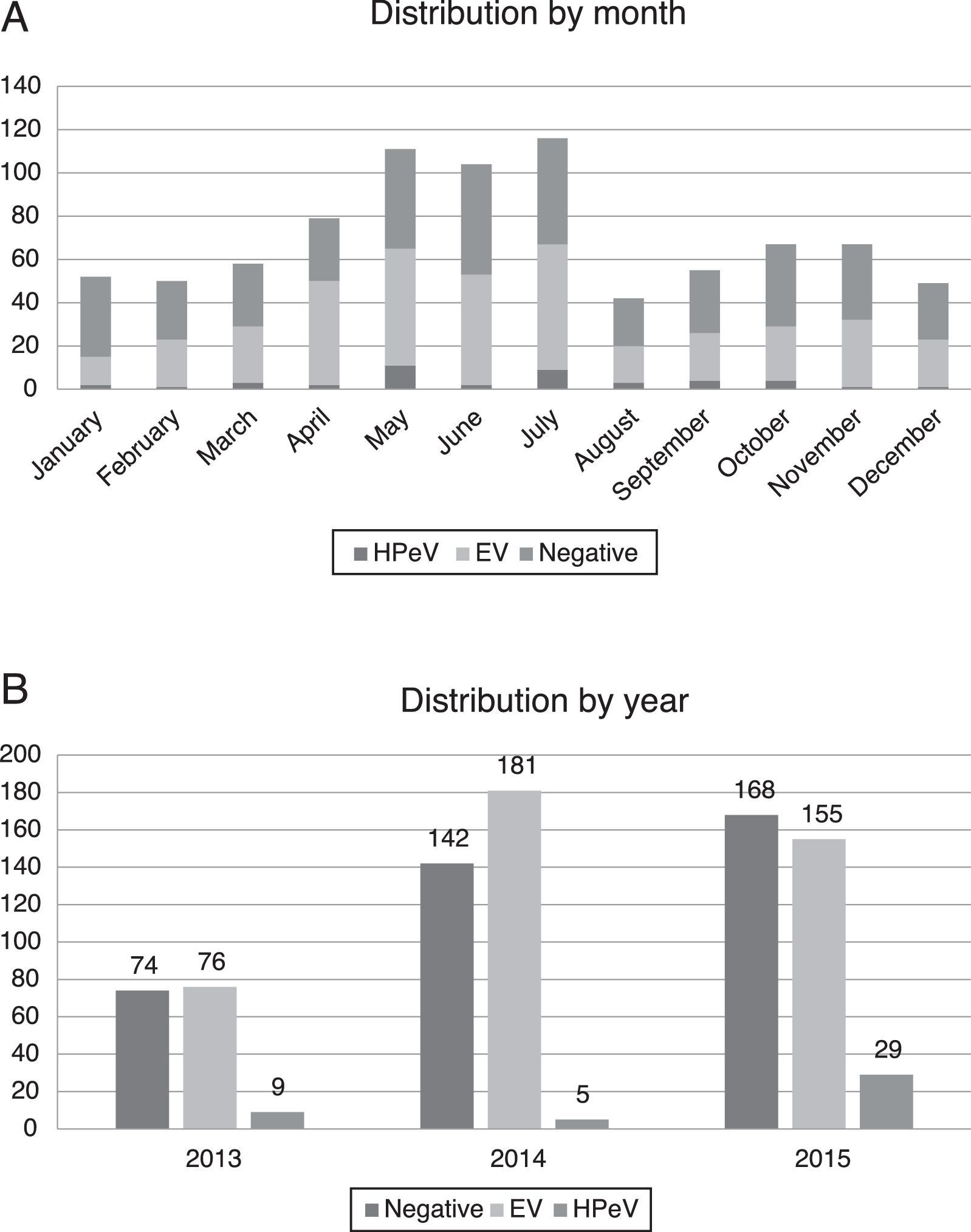
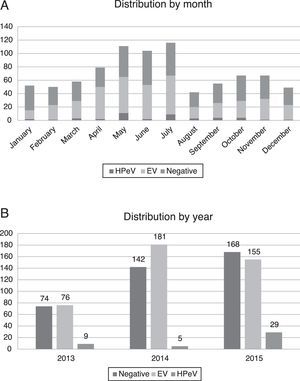
Most of the infections by HPeV took place in spring and summer, with peaks in the months of May (11 patients) and July (9 patients), although cases were detected year-round. There was a higher incidence in years 2013 (9/159 analysed samples, 5.66%) and 2015 (29/352, 8.29%), compared to 2014, when only 5 cases were detected (5/328, 1.52%) (Fig. 2).
All patients whose records included documentation of clinical manifestations presented with fever of more than 38°C, with a mean temperature of 38.8°C, a SD of 0.4°C and a maximum of 40.2°C. The mean duration of fever was 1.7 days (SD, 1.4), with a maximum of 6 days. The time elapsed from the onset of fever to the time of assessment ranged from 1 to 24h, with a mean of 8.3h (SD, 8.9).
Other clinical manifestations included irritability (45% of HPeV-positive patients), exanthema (18.6%), diarrhoea (14%) and, in smaller proportions, wheezing, enanthema and conjunctivitis.
Performance of blood tests revealed total white blood cell and neutrophil counts and levels of C-reactive protein and procalcitonin within normal ranges (Table 1). There were also no significant changes in CSF composition, as pleocytosis was not observed (save in 1 case) despite detection of HPeV. The levels of protein and glucose in CSF were also normal.
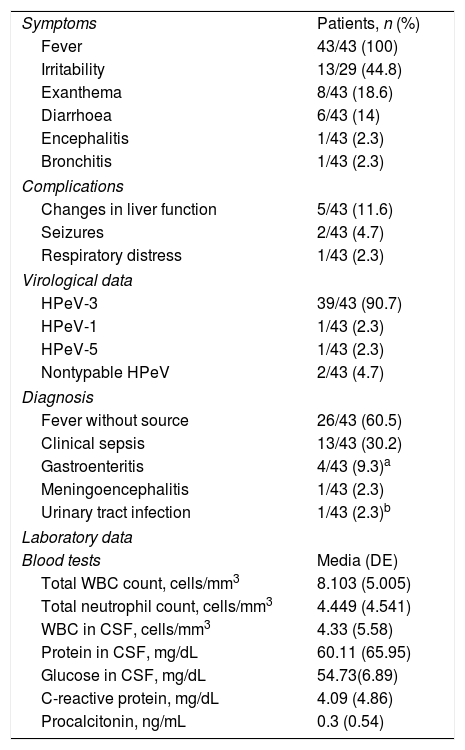
Clinical and laboratory characteristics of the 43 children with infection by parechovirus (HPeV).
| Symptoms | Patients, n (%) |
| Fever | 43/43 (100) |
| Irritability | 13/29 (44.8) |
| Exanthema | 8/43 (18.6) |
| Diarrhoea | 6/43 (14) |
| Encephalitis | 1/43 (2.3) |
| Bronchitis | 1/43 (2.3) |
| Complications | |
| Changes in liver function | 5/43 (11.6) |
| Seizures | 2/43 (4.7) |
| Respiratory distress | 1/43 (2.3) |
| Virological data | |
| HPeV-3 | 39/43 (90.7) |
| HPeV-1 | 1/43 (2.3) |
| HPeV-5 | 1/43 (2.3) |
| Nontypable HPeV | 2/43 (4.7) |
| Diagnosis | |
| Fever without source | 26/43 (60.5) |
| Clinical sepsis | 13/43 (30.2) |
| Gastroenteritis | 4/43 (9.3)a |
| Meningoencephalitis | 1/43 (2.3) |
| Urinary tract infection | 1/43 (2.3)b |
| Laboratory data | |
| Blood tests | Media (DE) |
| Total WBC count, cells/mm3 | 8.103 (5.005) |
| Total neutrophil count, cells/mm3 | 4.449 (4.541) |
| WBC in CSF, cells/mm3 | 4.33 (5.58) |
| Protein in CSF, mg/dL | 60.11 (65.95) |
| Glucose in CSF, mg/dL | 54.73(6.89) |
| C-reactive protein, mg/dL | 4.09 (4.86) |
| Procalcitonin, ng/mL | 0.3 (0.54) |
CSF, cerebrospinal fluid; SD, standard deviation; WBC, white blood cell.
As for the types of HPeV, the type detected most frequently was HPeV-3 (39/43, or 90.7% of cases) (Table 1). Coinfection was found in 2 patients. One of these patients (with HPeV-1) had Salmonella, which was detected by stool culture, and the other (with HPeV-3) had a urinary tract infection caused by Escherichia coli.
The most frequent final diagnosis, made on the basis of clinical manifestations, was FWS (26 patients; 60.5%), followed by clinical sepsis (13 patients; 30.2%), gastroenteritis (3 patients; 6.9%) and meningoencephalitis (1 patient). The latter developed neurologic sequelae, with persistence of epileptiform discharges on encephalography.
When we compared patients based on their diagnoses, we found that those with clinical sepsis were more likely to have significant liver involvement (28.6% vs 3.6%; p=.018). As expected, they required admission to the intensive care unit (ICU) more frequently than other patients (50% vs 19.2%, p=.043).
Ninety percent of the patients received antibiotic treatment, which was more frequent in newborns (100% vs 73%, p=.01). Twelve patients (26%) required admission to the ICU, of who 11 were aged less than 1 month.
The severe complications found in our patients were seizures in 2, respiratory distress in 1 and changes in liver function in 6 (with elevation of transaminases). None of the patients experienced coma, liver or heart failure or pleural effusion.
The comparison of newborns versus patients aged more than 1 month only revealed differences in the maximum body temperature (higher for age>1 month; 39.1°C vs 38.6°C; p=.01) and the haemoglobin concentration (higher in newborns; 14.5 vs 10.3mg/dL; p<.01).
All patients had favourable outcome and there were no deaths, although 1 patient required followup in the paediatric neurology department due to a persistent epileptiform pattern on electroencephalography.
DiscussionThis article presents the results of a prospective multicentre study conducted between 2013 and 2015, with collection of samples and clinical data for 845 patients presenting with clinical sepsis, meningitis, encephalitis or FWS, and evidence of infection associated with HPeV in 5.56% of the included patients (47 cases). This is the first study of these characteristics conducted nationwide and for a period of 3 years, and it demonstrated the presence of parechoviruses in Spain and their association with severe neurologic and systemic infections in young infants. In our case series, there were 2 hospitals were no cases of infection by HPeV were detected, located in the autonomous communities of Andalusia and Aragón. However, this may have been due to the low number of samples available for these hospitals, as opposed to the absence of parechovirus in these regions.
In our series, the circulation of HPeV exhibited a seasonal pattern, with its incidence peaking in spring and summer (Fig. 2). This temporal distribution differs slightly from the distributions reported in studies conducted in other countries, where the incidence peaked in summer and fall.8,9 We also found a biennial pattern in the circulation of HPeV-3, with a higher incidence in years 2013 and 2015 compared to 2014. Similar trends have been described before,10 although studies with a greater number of samples are needed to confirm the biennial pattern of HPeV-3.
Human parechoviruses are frequent aetiological agents in infections of early childhood, as previous molecular epidemiology studies have found that up to 90% of children aged less than 2 years have had infections by some type of HPeV, usually mild and manifesting with gastrointestinal or respiratory symptoms.11,12 However, a certain proportion of cases may present with more severe disease, with considerable impairment of general health and even requiring admission to the ICU. These cases correspond to systemic and neurologic infections (mainly clinical sepsis and encephalitis). Previous studies have reported HPeV-1 as the type detected most frequently, but HPeV-3 is the type associated with more severe forms of disease, such as meningitis or neonatal sepsis.13,14 In our series, HPeV-3 was also the type identified most frequently (90.7% of cases), although it must be taken into account that the inclusion criteria favoured the selection of patients with severe disease. Infection by HPeV-1 was only detected in one patient, who had gastroenteritis and also experienced seizures. However, we cannot be certain that all symptoms in this case were caused by parechovirus, as this patient also had a stool culture that was positive for Salmonella. The only patient infected by HPeV-5 presented with FWS in the neonatal period. These manifestations were similar to those described by van der Sanden et al.10 in a study conducted in the Netherlands over a period of 8 years in which HPeV-1 was associated with gastrointestinal symptoms, HPeV-3 with sepsis and HPeV-5 with gastrointestinal symptoms, fever and exanthema. In this same study, HPeV-3 was also the most frequent type, with a higher proportion compared to HPeV-1 (57% vs 35%). The difference found in our series and that of van der Sanden et al. in the genotypic distribution of HPeV is probably due to the inclusion criteria of both studies, suggesting that HPeV-3 may predominate in studies of hospitalised or severely ill patients and HPeV-1 in population-based studies.
The age distribution of patients with HPeV is distinct, with 83.7% of cases occurring in children aged less than 2 months, most frequently in neonates (55.8%). This has been described by other authors, such as Harvala et al., whose study in Scotland over an 8-year period only found HPeV infection in infants aged less than 3 months.15 The findings of another study conducted in Australia were similar, as 75% of the 118 children with infection by HPeV were less than 2 months of age.16 This age distribution differentiates HPeV from EV, which causes infections in individuals of all ages, and not only in young infants.17
The clinical presentation of HPeV infection included fever in all cases, although we found no characteristic duration or intensity of fever for these infections. Irritability was a frequent manifestation in our series, found in more than half of the cases, as was previously described by Vanagt et al. in a series of 89 patients in the Netherlands.18 Clinical sepsis was diagnosed in 30.4% of cases, and we found and association between this diagnosis and both changes in liver function and admission to the ICU. We found exanthema in 17.4%, a proportion that was similar to those described in other case series.19
The results of blood tests are generally nonspecific, with low white blood cell counts and C-reactive protein and procalcitonin levels, as occurs in other viral infections. The absence of pleocytosis is, however, significant, as it occurred in nearly all of the cases, consistent with the findings of other series, including a previous one published by our research group on infections by HPeV and EV in newborns.20,21 In most cases, the time elapsed from the onset of symptoms to performance of lumbar puncture may account for the low cell counts in CSF specimens. We ought to highlight the relevance of the absence of pleocytosis, as in these cases it would be convenient not to discard the CSF sample to assess for the presence of HPeV by PCR, which would make diagnosis possible. Thus, from a clinical standpoint, infection by HPeV presents as FWS and suspected sepsis in most patients, especially the youngest, and with poor general condition, and requires admission to the ICU in many cases, although most patients have favourable outcomes within a few hours, as observed in the cases described here. Meningoencephalitis was only diagnosed in one patient who experienced convulsive seizures and required followup due to the persistence of abnormal activity in the irritative area on encephalography recordings. However, there are studies that have described severe presentations, mainly with encephalitis, which were fatal in some cases.14,19,22
At present, there are no long-term data available on children with a history of infection by HPeV, but multiple studies have reported favourable outcomes in the short term. Still, in a retrospective review of 11 cases of HPeV infection in infants, Verboon-Maciolek et al.23 observed neurodevelopmental delays in 3. In our series, we also observed generally favourable outcomes of HPeV infection in the short term, despite most of our patients being infected by HPeV-3, which is associated with more severe disease. A recent study conducted on a small series of children, most of them born preterm, with severe encephalitis caused by HPeV described neurodevelopmental abnormalities in the long-term followup of these children.24
There is also no treatment available for infections by HPeV at present. Intravenous immunoglobulin therapy has been used in severe cases, for instance in the case reported by Wildenbeest et al., 25 in which a patient with dilated cardiomyopathy secondary to HPeV infection was treated successfully. Research is currently underway in the development of monoclonal antibodies against HPeV-1 and 2.26 However, more studies are required to introduce the use of monoclonal antibodies in clinical practice. Furthermore, it would be interesting to develop this type of treatment for HPeV-3, which is associated with the most severe infections. In our series, 90% of children received antibiotic treatment, a rate that went up to 100% in the newborn subset. The development of techniques for early diagnosis and of treatments specifically targeting this virus would help optimise the use of antibiotics, allowing their withdrawal once bacterial infection is ruled out. It is important to keep in mind that a small percentage of cases may involve bacterial coinfection.
We conclude that HPeV-3 is a frequent and relevant cause of FWS and clinical sepsis in young infants. It has a characteristic clinical presentation in infants aged less than 2 months, with fever, irritability, ill appearance and in some cases exanthema. Short-term outcomes are good, although some patients require admission to the ICU.
We believe that knowledge of the clinical characteristics of infection by HPeV and the use of molecular diagnostic methods in every hospital may help improve the management of patients with infection by this virus.
FundingThe study was funded by the Fondo de Investigaciones Sanitarias: AES (PI12-00904).
Conflicts of interestThe authors have no conflicts of interest to declare
The members of the Research Group on Infections by Enterovirus and Parechovirus in Children are: Martinez-Almagro C, Trallero G, del Amo E, García-Costa J, Omeñaca M, Sanbonmatsu-Gámez S, Pérez-Ruiz M, Santos-Muñoz MJ, García-García ML, Rey Cao S.
Please cite this article as: Martín del Valle F, Calvo C, Martinez-Rienda I, Cilla A, Romero MP, Menasalvas AI, et al. Características epidemiológicas y clínicas de los lactantes hospitalizados por infecciones por parechovirus humanos. Estudio prospectivo en España. An Pediatr (Barc). 2018;88:82–88.
Appendix A lists the members of the Research Group on Infections by Enterovirus and Parechovirus in Children.