Adalimumab (ADA), a monoclonal humanised anti-TNF antibody, is usually prescribed as a second-line treatment in paediatric Crohn's disease (CD) patients who have become unresponsive or developed intolerance to infliximab (IFX). In the case series reported, more than 70% of patients had initially been treated with IFX. Data on short- and long-term efficacy of ADA in anti-TNF naïve patients is limited. The aim of this study is to describe our experience with ADA as a first-line anti-TNF in paediatric CD patients.
Material and methodsThis is a multicentre retrospective study including anti-TNF naïve paediatric CD patients treated with ADA as first-line anti-TNF.
ResultsSixty-two patients (34 males), with a mean age of 13.0±2.4 years and a disease duration of 7.3 (IQR 2.7–21) months were included. Median wPCDAI was 35 (IQR 24.3–47.5). Fifty-eight out of 62 (93.5%) were on combo therapy at baseline. Clinical remission at week 12 was achieved in 50 out of 62 (80.6%) and in 57 out of 60 (95.0%) at week 52. Eight patients (13%) reported adverse events. Mean height, growth rate and BMI z-scores improved significantly between baseline and week 52, especially in patients with growth failure.
ConclusionsADA treatment leads to lasting clinical remission in anti-TNF naïve paediatric patients with CD. ADA significantly improved growth rate in children with CD who had growth delay at baseline. Some patients remain in remission for prolonged time periods under monotherapy; however, some patients would require dose escalation.
Adalimumab (ADA), anticuerpo anti-TNF-α monoclonal recombinante de origen humano, generalmente se emplea como tratamiento de segunda línea en niños con enfermedad de Crohn (EC) que no han respondido o han perdido respuesta a infliximab (IFX). En las series publicadas más del 70% de los pacientes habían sido tratados inicialmente con IFX. Los datos sobre la eficacia a corto y a largo plazo de ADA en pacientes naïve a anti-TNF son muy limitados. El objetivo del presente estudio es describir nuestra experiencia con ADA como tratamiento anti-TNF de primera línea en niños con EC.
Material y métodoEstudio multicéntrico, retrospectivo que incluye pacientes con EC tratados con ADA como anti-TNF de primera línea.
ResultadosSe incluyeron 62 pacientes (34varones) con una edad media de 13,0±2,4años, un tiempo de evolución de la enfermedad de 7,3meses (RIQ 2,7-21) y un wPCDAI de 35puntos (RIQ 24,3-47,5). En el momento de comenzar ADA, 58 pacientes (93,5%) estaban recibiendo tratamiento inmunomodulador. A las 12semanas de tratamiento el 80,6% (50/62) habían alcanzado la remisión clínica, así como el 95% (57/60) a las 52semanas. Ocho pacientes (13%) presentaron efectos adversos. Se constató un incremento significativo de los z-scores de talla, velocidad de crecimiento e índice de masa corporal (IMC) a las 52semanas de tratamiento, en especial en aquellos con retraso de crecimiento.
ConclusionesEl tratamiento con ADA favorece una remisión clínica prolongada en pacientes naïve a anti-TNF. El tratamiento con ADA mejora la velocidad de crecimiento en niños con EC y retraso de crecimiento al inicio del tratamiento.
Crohn's disease (CD) is a chronic disorder characterised by inflammation of the gastrointestinal tract. Its overall incidence and prevalence in children has increased in recent years: the incidence of CD in Spain has risen from 0.53 to 1.7 per 100,000 individuals <18 years of age in the last 14 years.1,2 Growth failure and delayed puberty are two specific paediatric features. Exclusive enteral nutrition (EEN) and corticosteroids are effective for the induction of clinical remission in children with CD. Anti-TNF therapy is recommended for inducing remission in children with active steroid-refractory disease, for inducing and maintaining remission in children with chronically active luminal CD despite prior optimised immunomodulatory therapy (azathioprine (AZA), mercaptopurine (MP) or methotrexate (MTX)) and as primary induction and maintenance therapy for children with active perianal fistulising disease in combination with appropriate surgical intervention.3
Infliximab (IFX) and adalimumab (ADA) are chimeric human-murine and fully humanised monoclonal antibodies, respectively, which specifically bind to tumour necrosis factor alpha (TNFα). Several studies have shown significant efficacy of IFX in the treatment of CD through sustaining clinical remission and improving overall clinical benefit.4 IFX is the usual first-line anti-TNF administered as therapy in children. ADA was approved later than IFX, and the demonstration of its efficacy in paediatric populations has been based mainly on retrospective studies and just one clinical trial (IMAgINE 1).5–12 In clinical practice, ADA is usually administered in patients who have lost response or developed intolerance to IFX.13 ADA has also shown its efficacy as first-line anti-TNF therapy in adults. Literature about the administration of ADA to paediatric patients with CD is scarce. Moreover, evidence for the short- and long-term efficacy of ADA in anti-TNF naïve patients is even more limited.14
The aim of the present study was to characterise the effectiveness and safety of ADA in achieving clinical remission and maintaining it over time in an anti-TNF-naïve population of paediatric patients with CD.
Material and methodsThree tertiary referral hospitals participated in this multicentre retrospective study. Clinical data from all paediatric CD patients under 18 years of age who had received ADA as first-line anti-TNF treatment were reviewed. All patients were naïve to anti-TNF treatment but might have previously been treated with corticosteroids and/or immunomodulators (AZA, MP, or MTX).
Diagnosis of Crohn's disease was established according to the revised Porto Criteria.15 Infection with tuberculosis was ruled out using the Mantoux and QuantiFERON®-TB Gold In-Tube test (Cellestis Ltd.; Carnegie, VIC, Australia) tests prior to starting treatment. Disease phenotype was established according to the Paris Classification.16
Prior to inclusion, the authors (JMC, GPM, MNR and VMNL) assessed each case and discussed the indication of an anti-TNF: a total (4/4) consensus was needed for each patient to be included in this study. Exclusion criteria were: the presence of active infection; heart, renal or liver failure; and neurological or immunodeficiency disorders. The Haycock formula17 was used for the calculation of body surface area.
The effectiveness of treatment as regards the induction and maintenance of clinical remission was assessed using a paediatric-specific disease activity score, the weighted Paediatric Crohn's Disease Activity Index (remission if wPCDAI<12.5 points). Primary non-response was defined as lack of improvement of clinical signs and symptoms at week 12 after induction therapy (wPCDAI≥12.5),18 mild response as a change of more than 12.5 points from baseline wPCDAI and moderate for more than 37.5 points from baseline wPCDAI changes. In addition to wPCDAI other remission criteria were used.
Dose escalation was indicated in patients with loss of response, defined as recurrence of disease activity (wPCDAI≥12.5) during maintenance therapy after achieving an appropriate induction response.19 The wPCDAI was calculated at baseline, week 12 and week 52. Faecal calprotectin (FC, Calprest®, Eurospital, Trieste, Italy) was used to establish the degree of intestinal mucosal inflammation. Samples for the determination of FC were collected at home by the patient the day before and were delivered refrigerated to the laboratory for analysis. Normal FC levels were considered to be below 50μg/g of faeces.
ADA induction dose (1 dose every other week (EOW)) was established according to basal weight: 160mg and 80mg, or 80mg and 40mg for ≥40kg or <40kg body weight, respectively. The first two doses of ADA were injected at the hospital under medical supervision. Subsequent doses were administered at the patient's home by parents or at their primary care centre. The standard maintenance dose was 40mg EOW. C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), haemoglobin (Hb), haematocrit (HCT), platelets, white blood cells (WBC), serum albumin, and FC levels were measured during follow-up. CRP levels were also expressed as the ratio between the value obtained and the upper limit of normal (ULN). Weight and height were measured with the patient barefoot and in underwear. Growth velocity, weight, height and body mass index (BMI) z-scores were calculated using data from Spanish growth Charts.20 Severe growth failure was defined as a growth rate z-score<−2.5 and mild to moderate failure as a growth rate z-score between −1.0 and −2.5.3 Growth velocity was calculated according to Tanner stage and to bone age when delayed puberty was present. Serious Adverse Event (SAE) was defined as any adverse event or adverse reaction that results in death, life-threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity.
In a first analysis, steroid-free remission at week 12 and 52 was assessed. Secondly, the need for dose escalation and the effect of anti-TNF treatment on the growth rate were also evaluated.
Statistical analysisVariables with normal distributions were expressed as means and standard deviations (SD); those without normal distributions were expressed as medians and interquartile ranges (IQRs). Kolmogorov–Smirnov test was used to evaluate normality of the distribution. Student's t-test and Wilcoxon signed-rank test were used for paired samples and the chi-squared test was used to compare proportions. Logistic regression was used to determine predictors of response and dose escalation. A p value<0.05 was considered to be statistically significant.
Ethical considerationsInformed consent was obtained from all patients or their parents. The study was approved by the Ethics Committees of all participating centres.
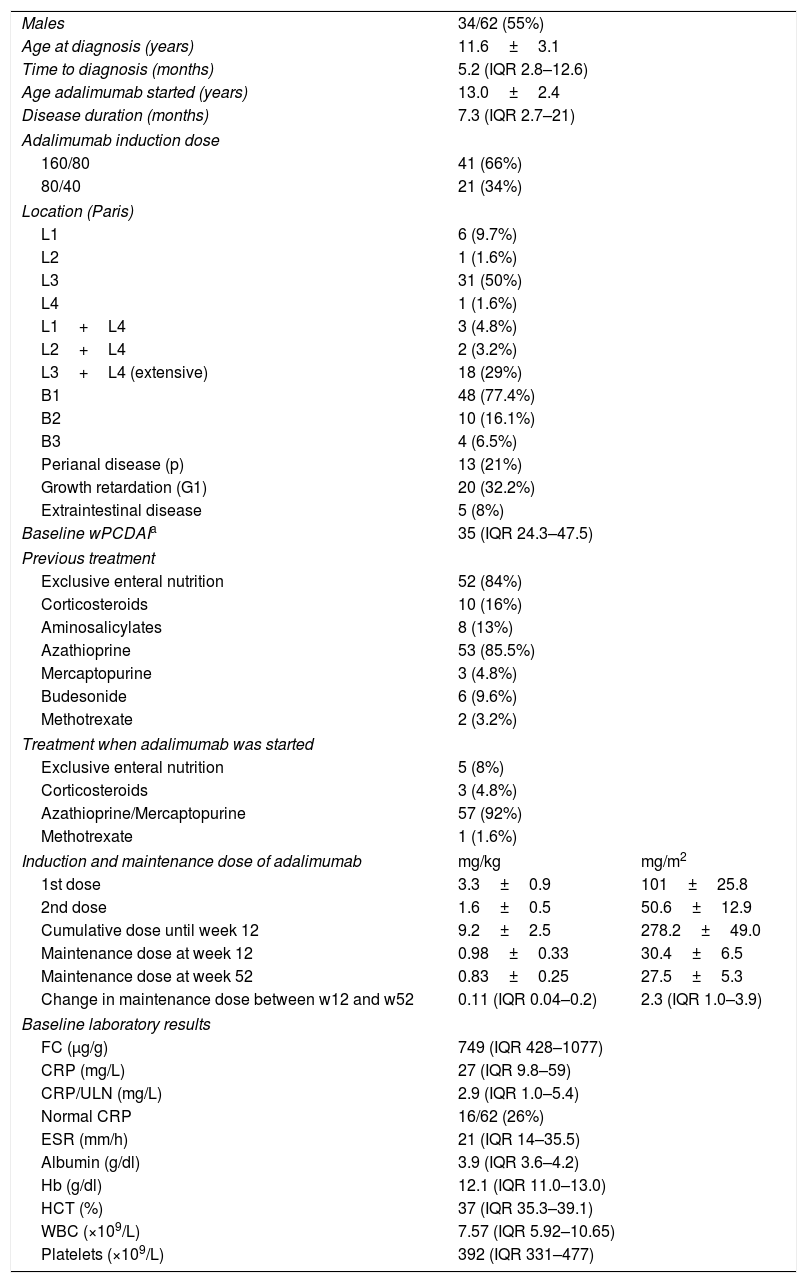
ResultsBaseline demographicsSixty-two children (34 males) with CD and naïve to anti-TNF treatment were included in the study. Demographic and clinical characteristics of patients at baseline are shown in Table 1. According to the Paris classification, 17 patients (27.4%) were in category A1a and 45 (72.6%) in A1b. Regarding disease location, 31 (50%) had ileocolonic involvement (L3) and 18 (29%) extensive disease (L3L4a/b). The most frequent behaviour phenotype was non-structuring non-penetrating disease (B1, 77.4%). Growth retardation (G1) was evidenced in 24 patients (38.7%). Most of the patients received EEN (52, 84%) and thiopurines (56, 90%) before starting ADA treatment. Ten patients (16%) had previously received corticosteroids. ADA was prescribed after failure of the induction therapy with EEN (n=4, 6.5%), after failure of the maintenance therapy with AZA after EEN success (n=41, 66%), because of severe extraintestinal manifestations (n=7, 11.3%), severe perianal disease (n=4, 6.5%), as top-down strategy in severe disease (n=3, 4.8%) or due to intolerance to previous treatment (AZA or MTX, n=6, 9.6%).
Baseline characteristics of patients treated with adalimumab.
| Males | 34/62 (55%) | |
| Age at diagnosis (years) | 11.6±3.1 | |
| Time to diagnosis (months) | 5.2 (IQR 2.8–12.6) | |
| Age adalimumab started (years) | 13.0±2.4 | |
| Disease duration (months) | 7.3 (IQR 2.7–21) | |
| Adalimumab induction dose | ||
| 160/80 | 41 (66%) | |
| 80/40 | 21 (34%) | |
| Location (Paris) | ||
| L1 | 6 (9.7%) | |
| L2 | 1 (1.6%) | |
| L3 | 31 (50%) | |
| L4 | 1 (1.6%) | |
| L1+L4 | 3 (4.8%) | |
| L2+L4 | 2 (3.2%) | |
| L3+L4 (extensive) | 18 (29%) | |
| B1 | 48 (77.4%) | |
| B2 | 10 (16.1%) | |
| B3 | 4 (6.5%) | |
| Perianal disease (p) | 13 (21%) | |
| Growth retardation (G1) | 20 (32.2%) | |
| Extraintestinal disease | 5 (8%) | |
| Baseline wPCDAIa | 35 (IQR 24.3–47.5) | |
| Previous treatment | ||
| Exclusive enteral nutrition | 52 (84%) | |
| Corticosteroids | 10 (16%) | |
| Aminosalicylates | 8 (13%) | |
| Azathioprine | 53 (85.5%) | |
| Mercaptopurine | 3 (4.8%) | |
| Budesonide | 6 (9.6%) | |
| Methotrexate | 2 (3.2%) | |
| Treatment when adalimumab was started | ||
| Exclusive enteral nutrition | 5 (8%) | |
| Corticosteroids | 3 (4.8%) | |
| Azathioprine/Mercaptopurine | 57 (92%) | |
| Methotrexate | 1 (1.6%) | |
| Induction and maintenance dose of adalimumab | mg/kg | mg/m2 |
| 1st dose | 3.3±0.9 | 101±25.8 |
| 2nd dose | 1.6±0.5 | 50.6±12.9 |
| Cumulative dose until week 12 | 9.2±2.5 | 278.2±49.0 |
| Maintenance dose at week 12 | 0.98±0.33 | 30.4±6.5 |
| Maintenance dose at week 52 | 0.83±0.25 | 27.5±5.3 |
| Change in maintenance dose between w12 and w52 | 0.11 (IQR 0.04–0.2) | 2.3 (IQR 1.0–3.9) |
| Baseline laboratory results | ||
| FC (μg/g) | 749 (IQR 428–1077) | |
| CRP (mg/L) | 27 (IQR 9.8–59) | |
| CRP/ULN (mg/L) | 2.9 (IQR 1.0–5.4) | |
| Normal CRP | 16/62 (26%) | |
| ESR (mm/h) | 21 (IQR 14–35.5) | |
| Albumin (g/dl) | 3.9 (IQR 3.6–4.2) | |
| Hb (g/dl) | 12.1 (IQR 11.0–13.0) | |
| HCT (%) | 37 (IQR 35.3–39.1) | |
| WBC (×109/L) | 7.57 (IQR 5.92–10.65) | |
| Platelets (×109/L) | 392 (IQR 331–477) | |
Remission<12.5; mild: 12.5–40; moderate: >40; severe: >57.5 points. Adapted from Ref. 18. wPCDAI: Weighted Paediatric Crohn's Disease Activity Index; CRP: C-reactive protein; ULN: upper limit of normal; Hb: haemoglobin; HCT: haematocrit; ESR: erythrocyte sedimentation rate; IQR: interquartile range; WBC: white blood cells; w: week; FC: faecal calprotectin.
During the study period (2008–2015), 89 patients with CD were treated with anti-TNF, 62 received ADA (69%) and 27 IFX (31%). Fifty-six out of 62 patients or their parents chose ADA as anti-TNF versus 5/27 of those who received IFX (90.3% vs 18.5%, p<0.0001). In 16 cases for IFX and in 6 patients for ADA the products were prescribed for different reasons (medical criteria, social problems, therapeutic compliance, etc.) without giving the option to choose.
Adalimumab induction and maintenance dosesThe doses used for induction and maintenance for remission are shown in Table 1. Maintenance dose were lower at week 52 compared to those on week 12 (p<0.0001) due mainly to the weight gain.
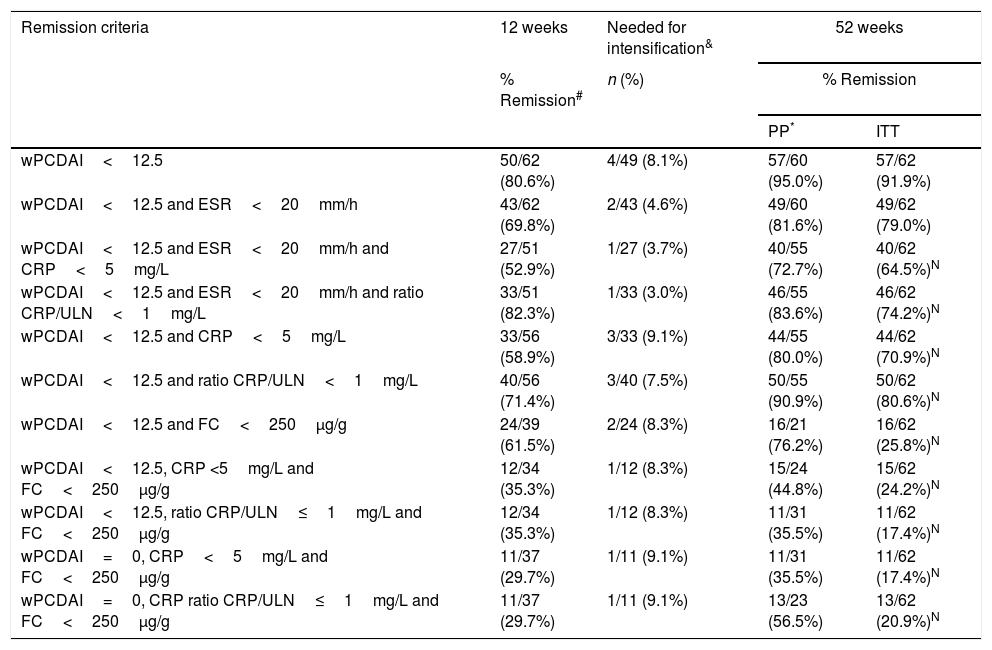
Clinical remission and clinical responseClinical remission was achieved at week 12 in 50 out of 62 (80.6%) and 3 patients showed a mild response (Table 2). Neither the wPCDAI, CRP, FC, ESR values, nor the induction dose per kg or per m2 of body surface or cumulative dose until week 12 of ADA per kg or per m2 of body surface area were predictive of response at week 12. Drug and antibodies levels were not requested.
Remission rates at weeks 12 and 52.
| Remission criteria | 12 weeks | Needed for intensification& | 52 weeks | |
|---|---|---|---|---|
| % Remission# | n (%) | % Remission | ||
| PP* | ITT | |||
| wPCDAI<12.5 | 50/62 (80.6%) | 4/49 (8.1%) | 57/60 (95.0%) | 57/62 (91.9%) |
| wPCDAI<12.5 and ESR<20mm/h | 43/62 (69.8%) | 2/43 (4.6%) | 49/60 (81.6%) | 49/62 (79.0%) |
| wPCDAI<12.5 and ESR<20mm/h and CRP<5mg/L | 27/51 (52.9%) | 1/27 (3.7%) | 40/55 (72.7%) | 40/62 (64.5%)N |
| wPCDAI<12.5 and ESR<20mm/h and ratio CRP/ULN<1mg/L | 33/51 (82.3%) | 1/33 (3.0%) | 46/55 (83.6%) | 46/62 (74.2%)N |
| wPCDAI<12.5 and CRP<5mg/L | 33/56 (58.9%) | 3/33 (9.1%) | 44/55 (80.0%) | 44/62 (70.9%)N |
| wPCDAI<12.5 and ratio CRP/ULN<1mg/L | 40/56 (71.4%) | 3/40 (7.5%) | 50/55 (90.9%) | 50/62 (80.6%)N |
| wPCDAI<12.5 and FC<250μg/g | 24/39 (61.5%) | 2/24 (8.3%) | 16/21 (76.2%) | 16/62 (25.8%)N |
| wPCDAI<12.5, CRP <5mg/L and FC<250μg/g | 12/34 (35.3%) | 1/12 (8.3%) | 15/24 (44.8%) | 15/62 (24.2%)N |
| wPCDAI<12.5, ratio CRP/ULN≤1mg/L and FC<250μg/g | 12/34 (35.3%) | 1/12 (8.3%) | 11/31 (35.5%) | 11/62 (17.4%)N |
| wPCDAI=0, CRP<5mg/L and FC<250μg/g | 11/37 (29.7%) | 1/11 (9.1%) | 11/31 (35.5%) | 11/62 (17.4%)N |
| wPCDAI=0, CRP ratio CRP/ULN≤1mg/L and FC<250μg/g | 11/37 (29.7%) | 1/11 (9.1%) | 13/23 (56.5%) | 13/62 (20.9%)N |
wPCDAI: weighted Paediatric Crohn's disease activity index; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; FC: faecal calprotectin; ULN: upper limit of normal.
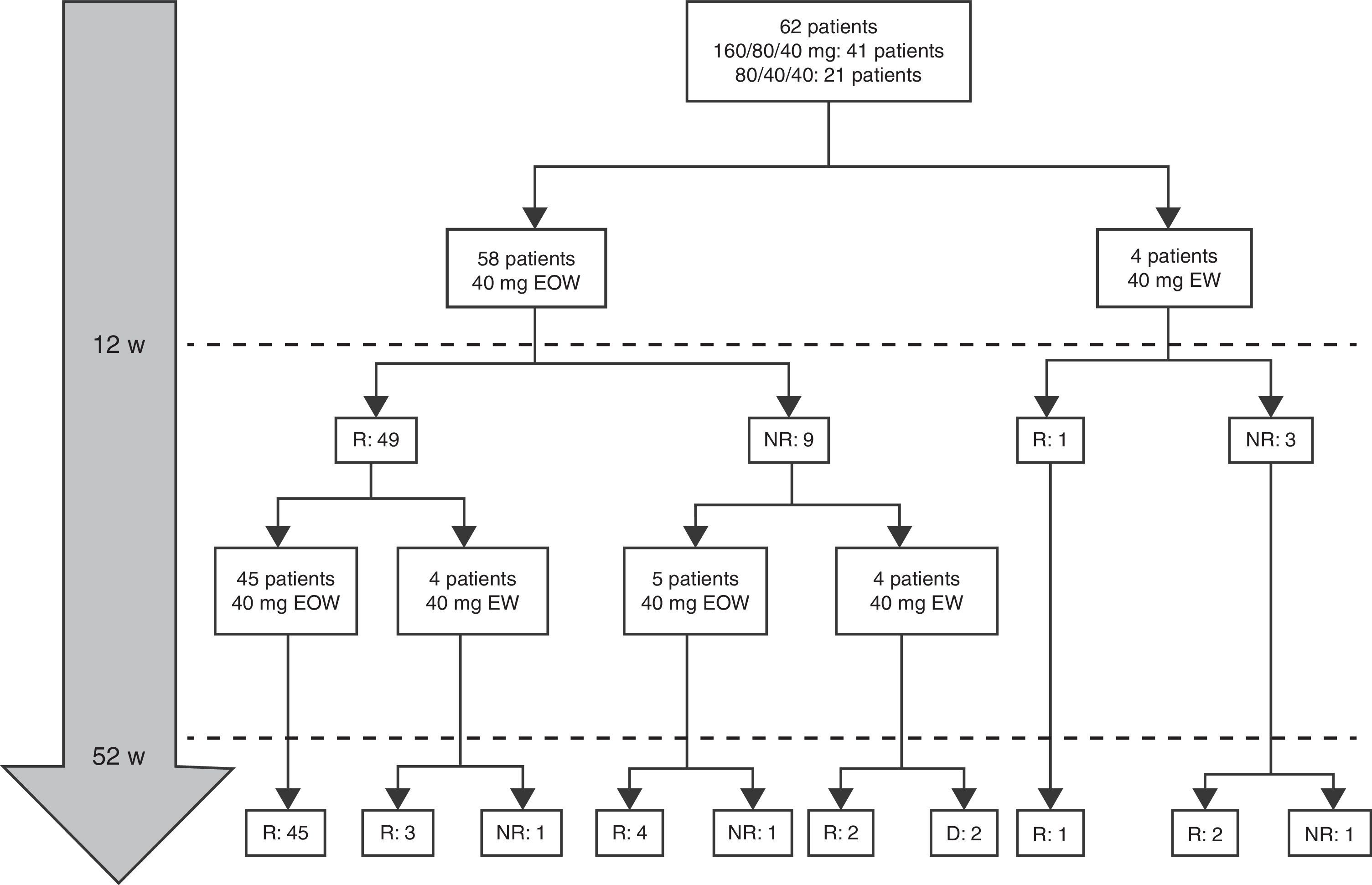
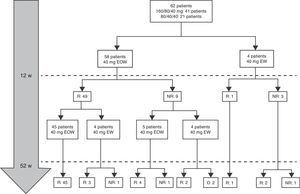
At week 52, 57 out of 60 patients (95%) were in clinical remission (Table 2) and 2 discontinued the treatment. Of these 57 patients, 10 (17.5%) had required treatment intensification (40mg/EW) during the first year of treatment with a median duration of 6 months (IQR 2.2–10.3). Of those patients requiring intensification during the first year of treatment, 66.7% were in remission at week 52 (Fig. 1).
Combo versus monotherapyAt baseline, 58 out of 62 patients (93.5%) were on combo therapy (Table 1). However, at 12 months of follow-up, only 23 patients (14 girls) were on combo therapy and 39 (63%) did not after 7 months (IRQ 4–11). The median duration of combination therapy was 12 months (IQR 6–14). ADA was discontinued in ten patients after 8.1 months (IQR 6.5–20.2). Two patients discontinued treatment with ADA from week 12, one for primary failure (serum ADA trough levels >12μg/ml and negative antibodies) and in the other case by development of anti-ADA antibodies forcing switch to IFX with good outcome. The reasons for stopping ADA were clinical remission continuing monotherapy (7 cases); adverse events (1 case) and treatment failure (2 cases).
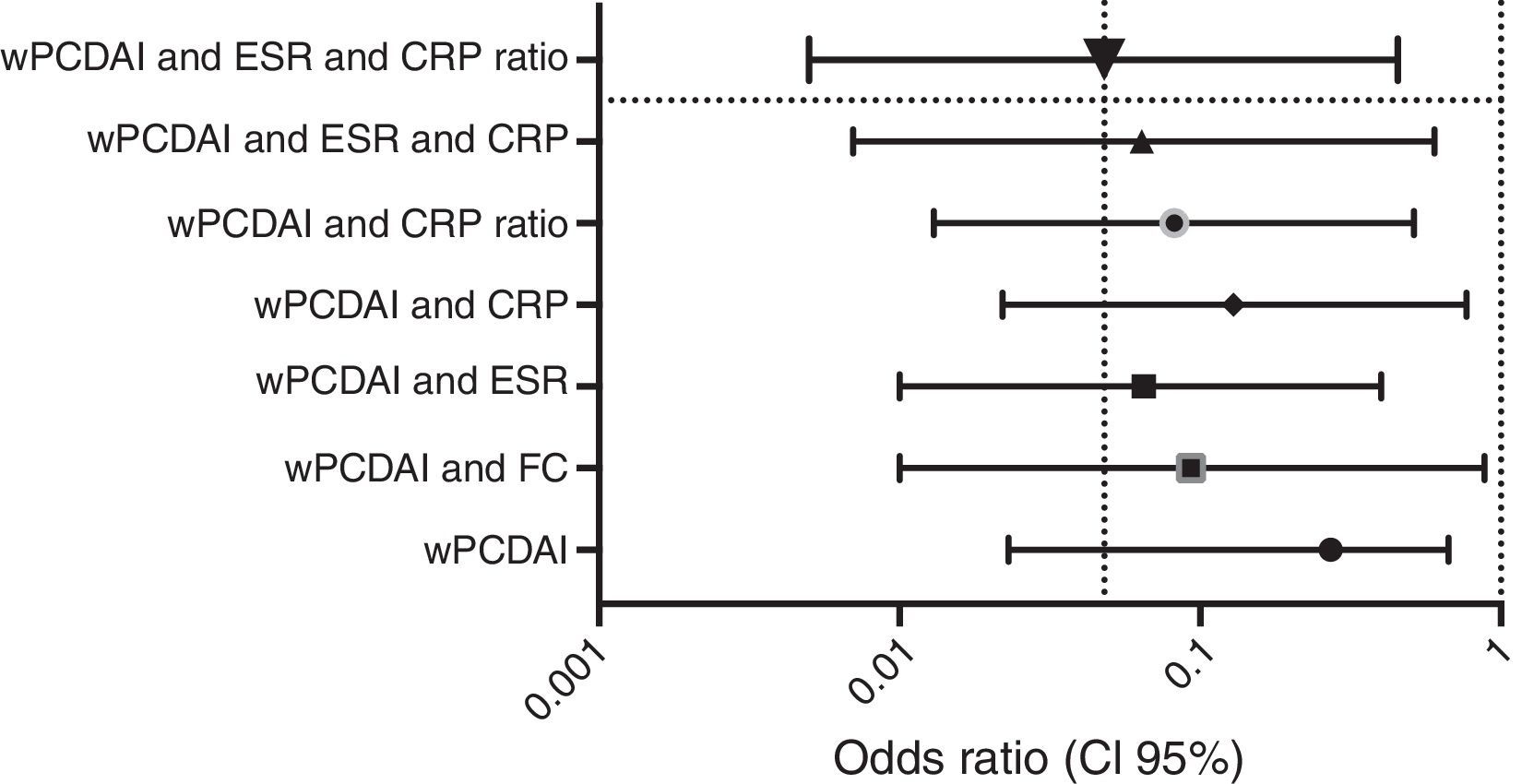
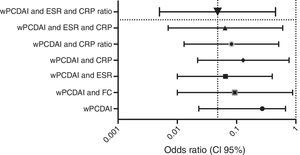
Dose escalationThe dose escalation was necessary in 16 of 62 cases (25.8%), 12 of them (75%) during the first year of treatment, at a mean of 12.9 weeks (IQR 8.4–17.7) after ADA was started. Twelve out of 16 patients (75%) were on combo therapy at the moment of intensification. Of them, 11 patients (68.7%) returned to the standard schedule at the end of the follow-up period. The median time during which they required ADA weekly was 5.9 months (IQR 2.1–10.5). None of the baseline characteristics were associated with dose escalation before week 12. However, remission criteria followed at week 12 were predictive of dose escalation during the following 40 weeks. wPCDAI<12.5 and ESR<20mm/h and ratio CRP/ULN<1 [OR 0.048 (0.005–0.455), p=0.008] was the best predictive remission criteria for not needing treatment intensification during the first year of treatment (Fig. 2).
Adverse eventsA total of 3103 injection doses were given over an average of 15.7 months (IQR 8.1–33) of treatment with a median of 42 doses (IQR 30–61) per patient and a median follow up of 20.7 months (IQR 14.2–33.5). Eight patients (13%) reported adverse events (AEs): recurrent episodes of amaurosis fugax (1), psoriasis (1), tremor (1), depressive syndrome (1) and mild reactions at the injection site (5). Only one patient had to discontinue ADA due to moderate pain at the injection site. There were no reports of allergic reactions, serious infections, such as tuberculosis or any other opportunistic infections, or malignancies.
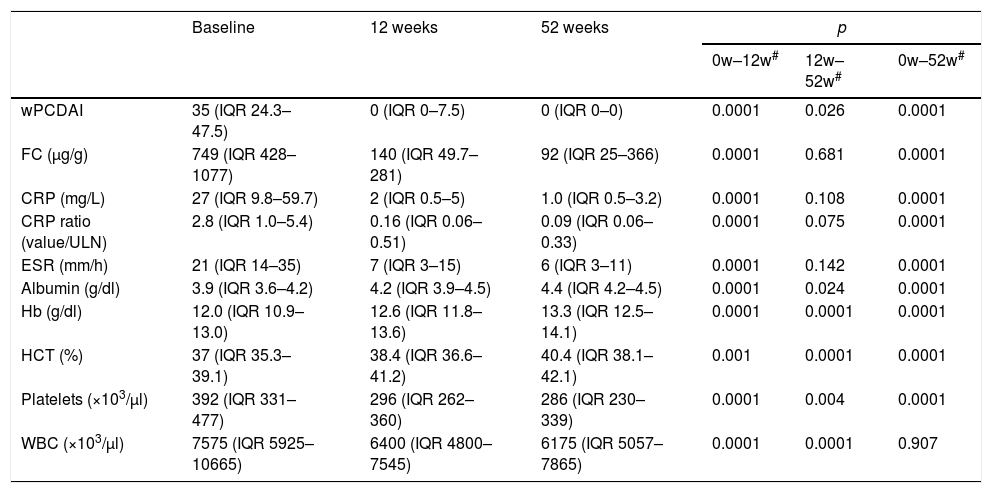
Changes in laboratory biomarkers during treatmentCRP, CRP ratio, ESR, WBC, platelet count and FC levels decreased significantly at weeks 12 and 52, and Hb, Htc and serum albumin were significantly higher (Table 3). FC data were collected from 23 patients at week 52; 12 of the 23 (52%) had FC levels less than 250μg/g and 8/23 (35%) lower than 50μg/g.
Outcome of wPCDAI and laboratory parameters after adalimumab treatment.
| Baseline | 12 weeks | 52 weeks | p | |||
|---|---|---|---|---|---|---|
| 0w–12w# | 12w–52w# | 0w–52w# | ||||
| wPCDAI | 35 (IQR 24.3–47.5) | 0 (IQR 0–7.5) | 0 (IQR 0–0) | 0.0001 | 0.026 | 0.0001 |
| FC (μg/g) | 749 (IQR 428–1077) | 140 (IQR 49.7–281) | 92 (IQR 25–366) | 0.0001 | 0.681 | 0.0001 |
| CRP (mg/L) | 27 (IQR 9.8–59.7) | 2 (IQR 0.5–5) | 1.0 (IQR 0.5–3.2) | 0.0001 | 0.108 | 0.0001 |
| CRP ratio (value/ULN) | 2.8 (IQR 1.0–5.4) | 0.16 (IQR 0.06–0.51) | 0.09 (IQR 0.06–0.33) | 0.0001 | 0.075 | 0.0001 |
| ESR (mm/h) | 21 (IQR 14–35) | 7 (IQR 3–15) | 6 (IQR 3–11) | 0.0001 | 0.142 | 0.0001 |
| Albumin (g/dl) | 3.9 (IQR 3.6–4.2) | 4.2 (IQR 3.9–4.5) | 4.4 (IQR 4.2–4.5) | 0.0001 | 0.024 | 0.0001 |
| Hb (g/dl) | 12.0 (IQR 10.9–13.0) | 12.6 (IQR 11.8–13.6) | 13.3 (IQR 12.5–14.1) | 0.0001 | 0.0001 | 0.0001 |
| HCT (%) | 37 (IQR 35.3–39.1) | 38.4 (IQR 36.6–41.2) | 40.4 (IQR 38.1–42.1) | 0.001 | 0.0001 | 0.0001 |
| Platelets (×103/μl) | 392 (IQR 331–477) | 296 (IQR 262–360) | 286 (IQR 230–339) | 0.0001 | 0.004 | 0.0001 |
| WBC (×103/μl) | 7575 (IQR 5925–10665) | 6400 (IQR 4800–7545) | 6175 (IQR 5057–7865) | 0.0001 | 0.0001 | 0.907 |
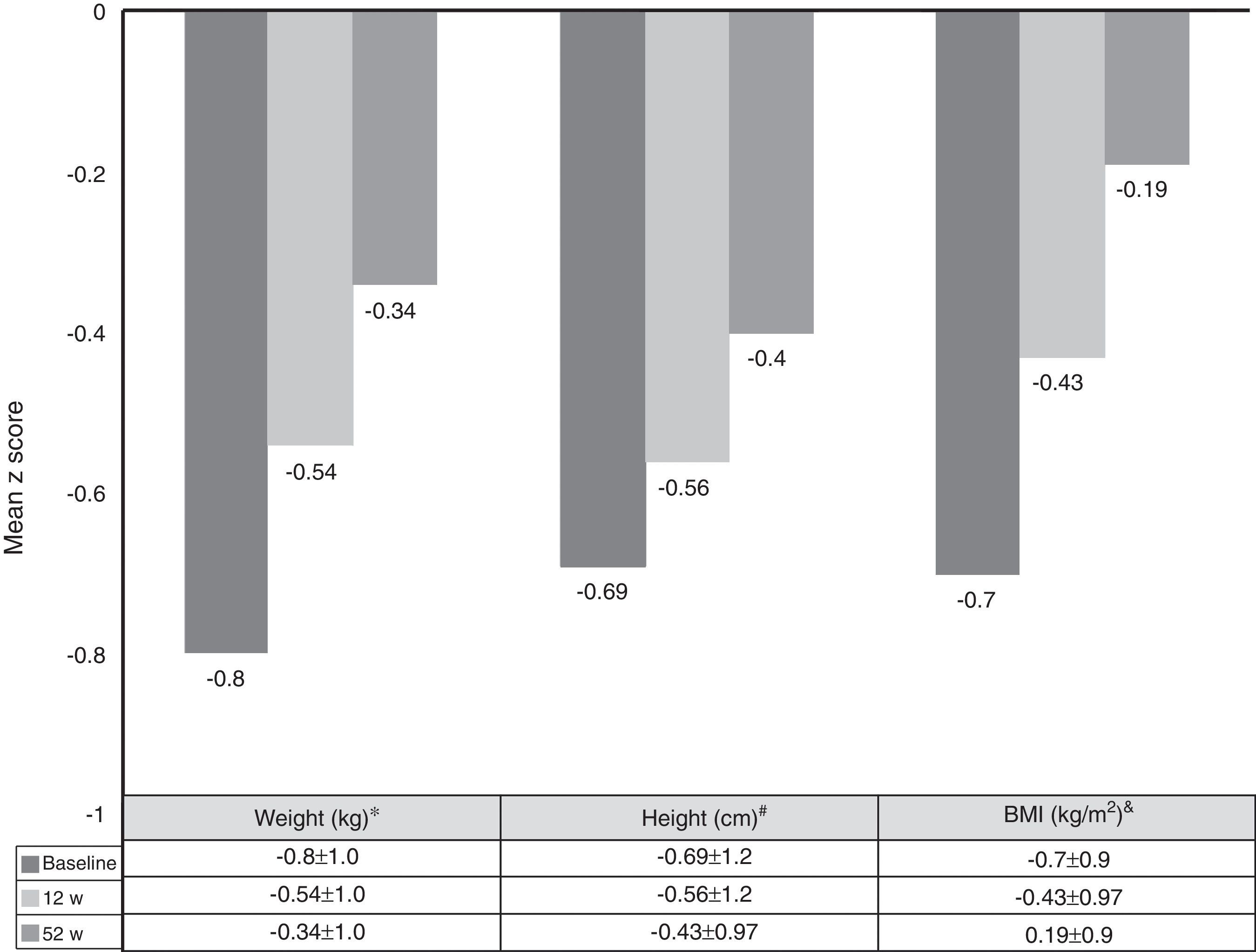
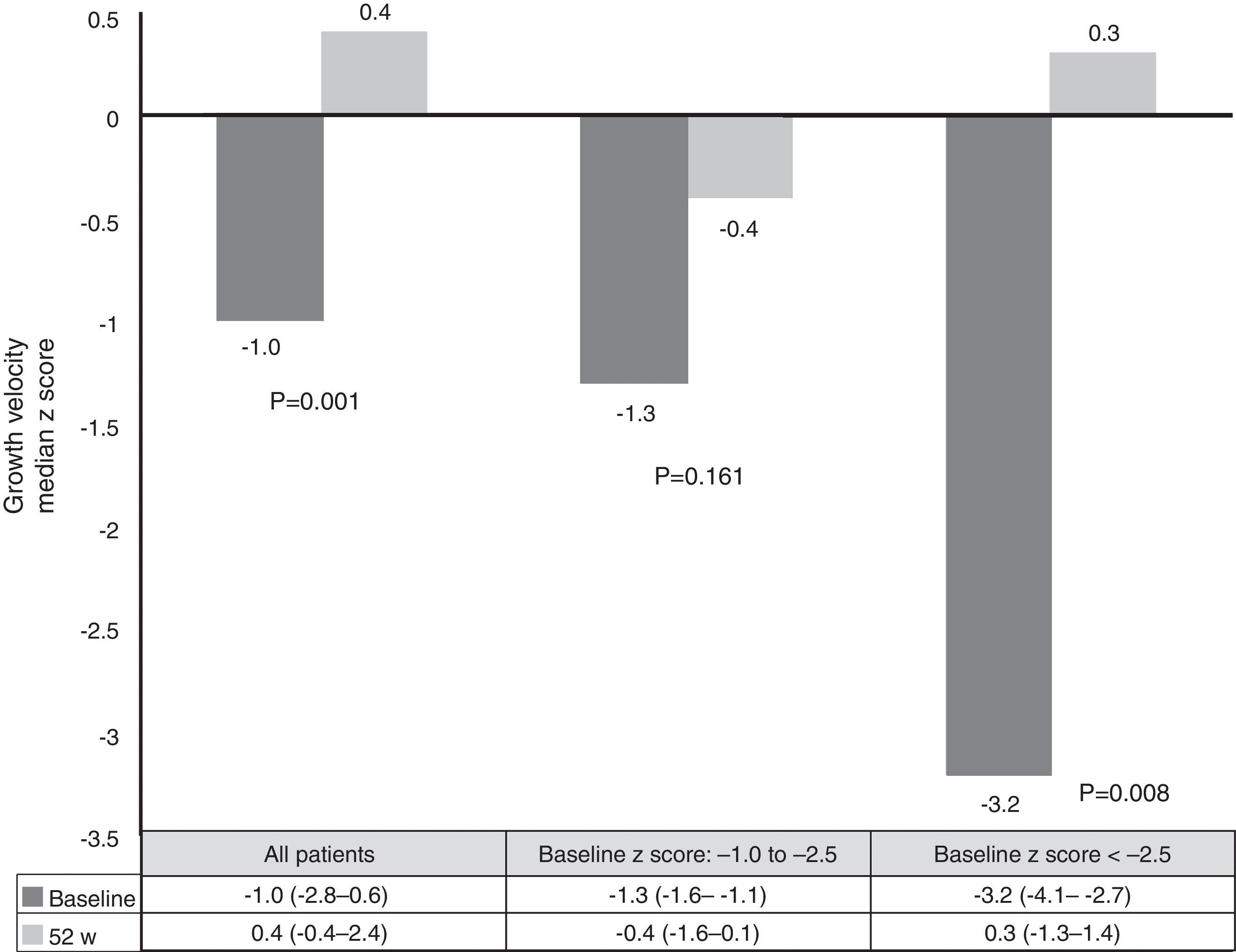
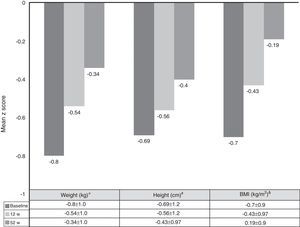
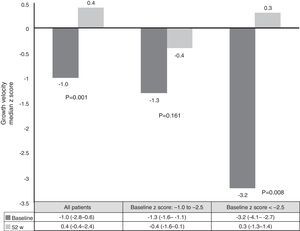
Growth rate and BMI were measured at the start of treatment with ADA and after 52 weeks of follow-up. Mean height, growth rate and BMI z-scores improved significantly between baseline and week 52 (Fig. 3). At it shown in Fig. 4, ADA treatment significantly improved z-score growth rate in children with CD especially in those with severe growth failure at baseline.
Changes in weight, height and BMI z scores from baseline to week 52. *BL versus 12w: p=0.0001; 12w versus 52w: p=0.01; BL versus 52w: p=0.0001. #BL versus 12w: p=0.005; 12w versus 52w: p=0.0001; BL versus 52w: p=0.0001. &BL versus 12w: p=0.0001; 12w versus 52w: p=0.804; BL versus 52w: p=0.039.
Literature concerning treatment with ADA in anti-TNF naïve paediatric CD patients is very scarce.21 Our data reveal promising results in terms of effectiveness, safety and growth as shown by short- and long-term clinical remission rates after 12 and 52 weeks of treatment. In a study conducted by Hyams et al.,10 including 105 infliximab-naïve patients, a remission rate of 56.9% at week 26 and 45.1% at week 52 was achieved in the high-dose (40mg EOW) group compared with 35.2% at week 26 and 27.8% at week 52 in the low-dose (20mg EOW) group, this was the main reason for not using low ADA dose in our study. Another possible explanation for our better results is the shorter disease duration before the first ADA dose (median 7.3 months in our study, as opposed to 2.9 years in Hyams’ study). A recent prospective study included 12 naïve anti-TNF patients treated with ADA, with a disease progression of 9.4±2.7 months (range 8.6–15.7), remission rate at 9–12 months starting treatment with ADA was 67.5%, the percentage of patients with mucosal healing was 41.7% and with partial mucosal healing 25%.12 Several studies have demonstrated an inverse relationship between disease duration and treatment outcome.21,22
Our results provide further evidence for the hypothesis that ADA may give better results in anti-TNF naïve patients than in IFX-experienced patients. Only one clinical trial has been carried out in a paediatric population, IMAgINE-1 (which includes the largest subgroup of anti-TNF naïve patients so far), and two trials in adult patients, CHARM23 and CLASSIC I,24 which have demonstrated positive results. Two prospective and several retrospective studies focused on the efficacy of ADA in paediatric populations which included a majority of patients with previous anti-TNF failure.5–7,9,21,22 The ADA induction doses in our series were 160/80mg, and 80/40mg for ≥40kg or <40kg body weight, respectively. The maintenance dose used for all patients was 40mg EOW. Our dosing schedule was similar to the higher ADA dose group in Hyams’ study, which was more successful for patients in remission than the lower dose group.10
The median duration of combination therapy in our series was 12 months (IQR 6–14). Although the data on this topic in paediatric populations is scarce, combination therapy during the first months of anti-TNF therapy may be associated with a lower rate of antibody development and loss of response. However, this benefit should be weighed against the potentially increased lymphoma risk with thiopurines.25
Malnutrition and growth failure are detrimental consequences in children with CD. The beneficial effect of anti-TNF on this issue has been reported in some studies.26–37 Nevertheless, some did not find any change in linear growth.26,30,31 One study reported nutritional and developmental effects of ADA in paediatric CD patients, but in this case all patients were IFX-experienced.33 Our study is the first report on growth and nutritional parameters effects of ADA in anti-TNF naïve patients. Furthermore, it confirms the beneficial effect in improving weight, height, BMI and growth rate over a period of one year. In our series, improvement in growth rate z-scores was found in patients with growth failure at baseline. These results are similar to those already published.10,26 One explanation for our results may be the steroid-sparing protocol followed in our study; only two patients used steroids concomitantly with ADA, because of severe extraintestinal manifestations.
Laboratory biomarkers play an important role in the follow-up of paediatric CD patients. Even if they are not as sensitive or specific as faecal biomarkers, they are very useful, in combination with clinical signs, to predict mucosal inflammation.35 The effects of ADA on Hb, HCT, WBC, platelet and albumin levels have also been described. All parameters showed a slow but progressive normalisation, maintaining normal levels until the end of the follow-up. With regard to safety, our experience showed a low rate of major AEs, including no malignancy, opportunistic infections, or surgical interventions. These findings are in accordance with other reports.5–7,9,26 By contrast, two studies described an incidence of 10%–43% of injection site reactions and 20% of serious AEs.10,12
In our series, 12 out of 62 patients (19.3%) had to intensify treatment to 40mg of ADA weekly during the first year of treatment, well below that recently published by Dubinsky et al.38 In this series (from the IMAgINE 1 study), 44% had to be intensified after 12 weeks of treatment, 50.5% of the low-dose group of adalimumab and 37.6% of those who were part of the high-dose group. At 52 weeks of follow-up, 18.8% of the low-dose group and 31.4% of the high-dose group were in remission. These percentages were higher in patients naïve to anti-TNF treated with high dose (15.4% and 44.4%) but much lower than those obtained in our series.
A previously undescribed contribution from paediatric series is the preference of children and their families to receive ADA subcutaneously injected at home instead of IFX administered at hospital. As specified in the European Crohn's and Colitis Organisation-European Society for Paediatric Gastroenterology and Nutrition (ECCO-ESPGHAN) consensus guidelines on the treatment of paediatric CD, considering IFX and ADA to be drugs of similar efficacy and safety profile for the treatment of anti-TNF naïve patients, these should be offered as options to the patient, depending on availability, route of administration, patient preferences, cost, and local regulations. Different series of adult patients with disease subsidiary to anti-TNF treatment have shown ease of use and time spent on administration as the two factors that most influence the choice of treatment.39,40 Furthermore, involving the patient and caregivers in choosing the drug could, apart from strengthening the doctor–patient relationship, allow greater patient responsibility in their treatment and self-care. This strategy, put into practice in our centres, has shown that 90% of our paediatric patients who have been offered the choice prefer ADA to the option of intravenous administration of IFX in hospital.
This study has a number of limitations, including small sample size, its retrospective nature, the lack of a control group, the short follow-up period, absence of drug levels and monitoring of mucosal healing with colonoscopy. However, this series provides important data: the effectiveness of ADA to normalise growth rate in paediatric patients with Crohn's disease and the predictive factors for dose escalation.
In conclusion, ADA as first-line anti-TNF therapy induces and maintains clinical remission in paediatric patients with CD. Moreover, ADA has a beneficial effect on nutritional and growth parameters, and is also well tolerated. Some patients maintain remission for prolonged time periods under monotherapy; however some patients in this situation would require dose escalation.
AuthorshipAll authors have made substantial contributions to all of the following:
- (1)
the conception and design of the study, or acquisition of data, or analysis and interpretation of data: Víctor Manuel Navas-López, Gemma Pujol Muncunill, Enrique Llerena, María Navalón Rubio and J. Martin-de-Carpi
- (2)
drafting the article or revising it critically for important intellectual content: Víctor Manuel Navas-López, Gemma Pujol Muncunill, Enrique Llerena, María Navalón Rubio, David Gil-Ortega, Vicente Varea-Calderón, Carlos Sierra Salinas and J. Martin-de-Carpi
- (3)
final approval of the version to be submitted: Víctor Manuel Navas-López, Gemma Pujol Muncunill, Enrique Llerena, María Navalón Rubio, David Gil-Ortega, Vicente Varea-Calderón, Carlos Sierra Salinas and J. Martin-de-Carpi.
This manuscript, including related data, figures and tables, has not been previously published and is not under consideration elsewhere.
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consentInformed consent was obtained from all individual participants included in the study.
Conflict of interestDr Victor Manuel Navas-López has received consulting fees and speakers’ fees from Abbvie; Dr María Navalon Rubio has received consulting fees and speakers’ fees from Abbvie; Dr Enrique Llerena declares that he has no conflict of interest. Dr Gemma Pujol declares that she has no conflict of interest; Dr David Gil-Ortega declares that he has no conflict of interest; Dr Vicente Varea-Calderón declares that he has no conflict of interest; Dr Carlos Sierra declares that he has no conflict of interest. Dr Javier Martín-de-Carpi has received consulting fees and speakers’ fees from Abbvie.
The authors would like to thank Pablo Vivanco Jódar (PhD, Meisys) for his assistance in writing the manuscript.
Please cite this article as: Navas-López VM, Muncunill GP, Llerena E, Rubio MN, Gil-Ortega D, Varea-Calderón V, et al. A real-world study focused on the effectiveness and safety of adalimumab as first-line anti-TNF treatment for paediatric Crohn's disease. An Pediatr (Barc). 2018;88:89–99.