Rumination syndrome is an uncommon gastrointestinal functional disorder that may be difficult to diagnose, as not many physicians are aware of this condition. In many cases, patients undergo numerous tests and are prescribed several treatments based on erroneous diagnoses. When the correct diagnosis is eventually made, therapy for the syndrome can be difficult and complex because of its multifactorial nature. The aim of this study was to present our experience with this condition, by presenting an analysis of the clinical, diagnostic, and therapeutic data of our patients.
Patients and methodA prospective and retrospective study was conducted on all cases of rumination syndrome diagnosed between January 2010 and May 2016 in patients attending the Paediatric Gastroenterology Departments of two hospitals: Consorci Sanitari de Terrassa and Hospital Materno-Infantil Vall d’Hebron (Barcelona, Spain).
ResultsThe analysis included 12 patients, with a mean age at the onset of symptoms of 9 years and 1 month, and the mean time period to make the diagnosis was 2 years and 3 months. A mean of 8.1 complementary tests were carried out before establishing the diagnosis. In 10 of the 12 patients, some type of treatment had been given before the diagnosis of rumination syndrome, but was unsuccessful in all cases. Ten of our patients underwent the novel, experimental biofeedback therapy.
ConclusionsDue to the limited knowledge of this condition among attending professionals in terms of the clinical presentation, diagnosis, and treatment, patients with rumination syndrome are often misdiagnosed and undergo numerous avoidable complementary tests, and invasive, costly treatments.
El Síndrome de rumiación es un trastorno gastrointestinal funcional poco común. De diagnóstico difícil, por el desconocimiento del mismo dentro del colectivo médico, acaba conllevando la realización de múltiples pruebas complementarias, la aplicación de diferentes tratamientos, y diagnósticos tardíos o erróneos, en la mayoría de los casos. Su tratamiento es difícil y complejo dada su naturaleza multifactorial. El objetivo de este estudio es presentar nuestra casuística analizando sus datos clínicos, diagnósticos y terapéuticos.
Pacientes y métodoEstudio descriptivo y retrospectivo de todos los casos diagnosticados entre enero del 2010 y mayo del 2016, controlados en las unidades de Gastroenterología Pediátrica del Consorci Sanitari de Terrassa y del Hospital Materno-Infantil Vall d’Hebron.
ResultadosSe analizó a un total de 12 pacientes. Una media de edad al inicio de los síntomas de 9 años y un mes, con un tiempo medio de evolución antes de llegar al diagnóstico de 2 años y 3 meses, y una media de pruebas complementarias realizadas hasta del diagnóstico de 8,1. En 10 de los 12 pacientes se había probado, antes del diagnóstico de rumiación, algún tipo de tratamiento que resultó ineficaz en todos los casos. Como novedad terapéutica, 10 de nuestros casos se sometieron a un tratamiento experimental de biofeedback.
ConclusionesDebido al conocimiento limitado de esta entidad, entre nuestros profesionales, en cuanto a su presentación clínica, diagnóstico y tratamiento, estos pacientes son frecuentemente mal diagnosticados y, a menudo, se ven sometidos a pruebas complementarias y tratamientos evitables, invasivos y costosos.
Rumination syndrome is an uncommon functional gastrointestinal disorder defined by the current Rome IV criteria as repeated regurgitation or expulsion of food that begins soon after ingestion of a meal, does not occur during sleep, is not preceded by retching or nausea and occurs in the absence of any known structural disease or eating disorder1 (Table 1). The food may then be rechewed, expelled or reswallowed by the patient.2,3
Diagnostic criteriaa for rumination syndrome (Rome IV).
| Must include all of the following: |
| Repeated regurgitation and rechewing or expulsion of food that: |
| Begins soon after ingestion of a meal |
| Does not occur during sleep |
| Not preceded by retching |
| After appropriate evaluation, the symptoms cannot be fully explained by another medical condition. An eating disorder must be ruled out |
It may be associated with other complaints, such as abdominal pain, abdominal distension, heartburn, headache, dizziness and sleeping difficulties.1–3
In the past it was believed that it was more prevalent in patients with some form of intellectual disability, but it is currently known that it may occur in patients of any background. It may present at any age, and adolescents and women are the groups at highest risk.1,3
The prevalence of this disorder is unknown, as rumination is often kept hidden, so that parents may be unaware of the problem and thus not seek medical help.1–4
Rumination results from various aetiological and pathogenic factors. On one hand, there is an increase of intragastric pressure due to the voluntary but inadvertent contraction of abdominal and intercostal muscles, associated with the simultaneous relaxation of the lower oesophageal sphincter. There is also evidence suggesting that at the same time, the gastroesophageal junction is displaced into the thorax, all of which would explain the retrograde flow of gastric contents into the oesophagus.1–5
Although rumination is often triggered by stressors, this is not the case in many patients. An intercurrent infectious process may cause nausea and ‘vomiting’ that do not disappear when the infection is resolved. In other cases a traumatic psychosocial event, such as depression, anxiety disorder, obsessive-compulsive disorder, developmental delay, attention-deficit hyperactivity disorder or another psychiatric disorder may be recognised at the onset of rumination.1–4
The diagnosis may be challenging in some cases due to the lack of awareness of this disorder by physicians. Multiple diagnostic tests may be performed, most of them with normal findings, and different treatments prescribed that are usually ineffective, leading to delayed or incorrect diagnosis in most patients.2,3 As a result, families experience considerable anxiety, the patient experiences negative effects of avoidable treatment, and health care costs rise.
Direct observation of rumination episodes while the child eats is of vital importance and may be crucial in making the correct diagnosis avoiding the use of unnecessary diagnostic tests or treatments.
The differential diagnosis includes various gastrointestinal disorders, such as gastro-oesophageal reflux disease (GERD), gastroparesis, achalasia or bulimia nervosa, among others,1,2,4 although none of these diseases meet the Rome IV criteria for rumination disorder.1
Treatment is difficult and complex due to the multifactorial nature of the disorder, and usually involves both medical and behavioural interventions, most of them of limited efficacy.1–4
The aim of our study was to present our experience with this disorder, analysing data on clinical manifestations, diagnostic tests, prescribed treatments and their outcomes.
Patients and methodsWe conducted a retrospective descriptive study of all cases of rumination syndrome diagnosed in patients aged less than 18 years between January 2010 and May 2016 managed at the paediatric gastroenterology units of the Consorci Sanitari de Terrassa centres and the Hospital Universitari Vall d’Hebron.
We analysed the following variables: age of onset, time elapsed from onset to definitive diagnosis, number of diagnostic tests performed and treatments received, and, last of all, the presence of comorbidities or factors that may have acted as triggers for the disease.
In the statistical analysis, we summarised qualitative variables as absolute frequencies and percentages, and quantitative variables as means.
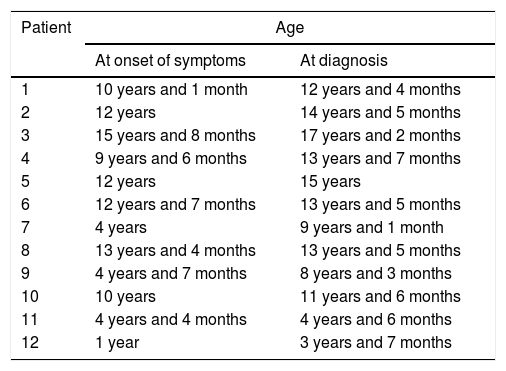
ResultsWe collected data for a total of 12 patients: 8 male (66.6%) and 4 female (33.3%). The mean age at onset of symptoms was 9 years and 1 month, with a mean time to diagnosis of 2 years and 3 months (Table 2).
Age at onset of symptoms and at diagnosis.
| Patient | Age | |
|---|---|---|
| At onset of symptoms | At diagnosis | |
| 1 | 10 years and 1 month | 12 years and 4 months |
| 2 | 12 years | 14 years and 5 months |
| 3 | 15 years and 8 months | 17 years and 2 months |
| 4 | 9 years and 6 months | 13 years and 7 months |
| 5 | 12 years | 15 years |
| 6 | 12 years and 7 months | 13 years and 5 months |
| 7 | 4 years | 9 years and 1 month |
| 8 | 13 years and 4 months | 13 years and 5 months |
| 9 | 4 years and 7 months | 8 years and 3 months |
| 10 | 10 years | 11 years and 6 months |
| 11 | 4 years and 4 months | 4 years and 6 months |
| 12 | 1 year | 3 years and 7 months |
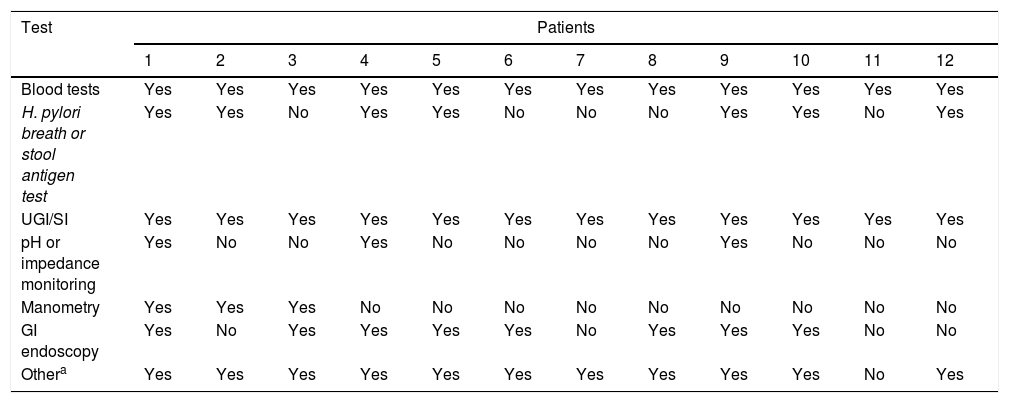
The diagnostic tests performed in all patients included blood tests (complete blood count, urea, creatinine, electrolyte panel, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase, alkaline phosphatase, total and direct bilirubin, glucose, total protein, albumin, ammonia, lactate, immunoglobulins, free T4, thyroid-stimulating hormone, ferritin, erythrocyte sedimentation rate, C-reactive protein, blood gases and coagulation factors) and upper GI and small bowel series (UGI/SI). The presence of Helicobacter pylori (H. pylori) was assessed through a breath test or stool antigen test in 7 patients (58.3%). In 3 patients (25%), pH or multichannel intraluminal impedance monitoring was performed to rule out GERD. Three patients (25%) were assessed by antroduodenal manometry and 8 (66.6%) by upper gastrointestinal endoscopy. Such supplementary tests were performed in all patients but one, in addition to other diagnostic tests that are not specified in Table 3: detection of specific IgE by RAST and/or prick test for assessment of food allergies, coeliac disease workup, measurement of faecal calprotectin, abdominal X-ray and/or ultrasound examination, abdominal CT and/or MRI, gastric emptying scan, head CT and/or MRI.
Diagnostic tests.
| Test | Patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Blood tests | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| H. pylori breath or stool antigen test | Yes | Yes | No | Yes | Yes | No | No | No | Yes | Yes | No | Yes |
| UGI/SI | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| pH or impedance monitoring | Yes | No | No | Yes | No | No | No | No | Yes | No | No | No |
| Manometry | Yes | Yes | Yes | No | No | No | No | No | No | No | No | No |
| GI endoscopy | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No |
| Othera | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
The mean number of diagnostic tests performed before the diagnosis was made was 8.1, with a maximum of 13 in 2 patients. One of these two patients had received an initial diagnosis of eosinophilic oseophagitis confirmed by endoscopy, and the other a diagnosis of recurrent nonspecific abdominal pain.
We ought to highlight the regrettable fact that in some patients, the same diagnostic tests were performed repeatedly, despite having clearly normal results. On the other hand, it is also worth noting that in one patient, only 2 diagnostic tests were performed (blood panel and UGI/SI), possibly because this was one of the latest cases diagnosed, when our degree of suspicion based on clinical manifestations alone was higher.
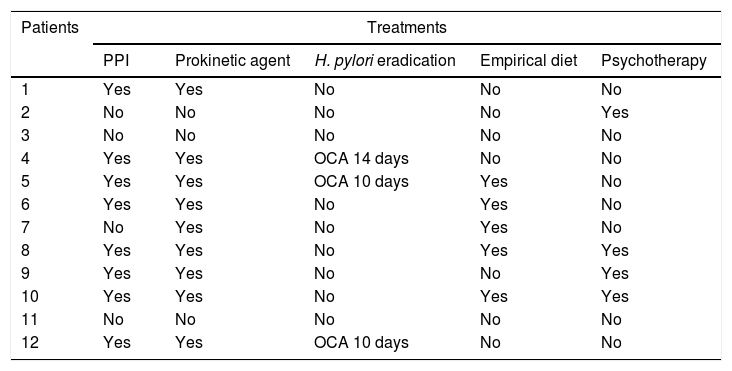
As can be seen in Table 4, some type of treatment was tried before or after the definitive diagnosis in 10 patients, and was ineffective in all: 9 patients received prokinetic agents (domperidone in 8 and metoclopramide in 1), 8 patients received proton pump inhibitors, 5 made dietary changes, going on empirical exclusion diets due to suspicion of some type of food intolerance, 3 patients received triple therapy for H. pylori eradication (omeprazole, clarithromycin and amoxicillin [OCA]) following detection of the pathogen by indirect methods (breath test or stool antigen test), and 4 patients received psychotherapy.
Treatments performed.
| Patients | Treatments | ||||
|---|---|---|---|---|---|
| PPI | Prokinetic agent | H. pylori eradication | Empirical diet | Psychotherapy | |
| 1 | Yes | Yes | No | No | No |
| 2 | No | No | No | No | Yes |
| 3 | No | No | No | No | No |
| 4 | Yes | Yes | OCA 14 days | No | No |
| 5 | Yes | Yes | OCA 10 days | Yes | No |
| 6 | Yes | Yes | No | Yes | No |
| 7 | No | Yes | No | Yes | No |
| 8 | Yes | Yes | No | Yes | Yes |
| 9 | Yes | Yes | No | No | Yes |
| 10 | Yes | Yes | No | Yes | Yes |
| 11 | No | No | No | No | No |
| 12 | Yes | Yes | OCA 10 days | No | No |
Patients received a mean of 2.4 different treatments. Only 2 of the patients in the study did not receive any treatment, as the clinical manifestations were specific enough for diagnosis.
Ten patients tried a new experimental treatment based on the use of biofeedback to develop control of abdominothoracic muscles.
Of the 10 patients treated with biofeedback, 7 improved after the 3 initial sessions, an improvement that was sustained at 3 and 6 months in three of them. The remaining patients are still being treated with this modality, so we do not have the corresponding outcome data.
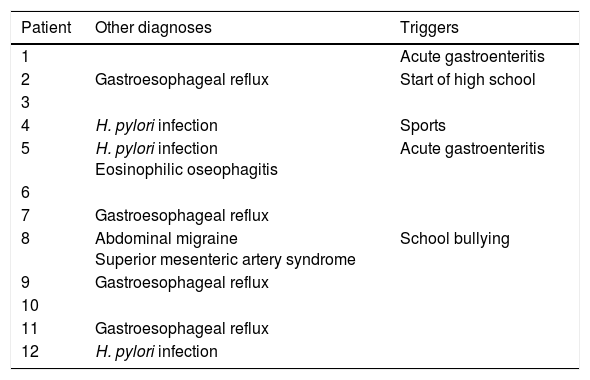
As for other diagnosed disorders and/or potential triggers, 10 patients received diagnoses of concomitant disorders before that of rumination syndrome, and 2 of these patients received 2 prior diagnoses each (Table 5).
Other diagnoses and symptom triggers.
| Patient | Other diagnoses | Triggers |
|---|---|---|
| 1 | Acute gastroenteritis | |
| 2 | Gastroesophageal reflux | Start of high school |
| 3 | ||
| 4 | H. pylori infection | Sports |
| 5 | H. pylori infection Eosinophilic oseophagitis | Acute gastroenteritis |
| 6 | ||
| 7 | Gastroesophageal reflux | |
| 8 | Abdominal migraine Superior mesenteric artery syndrome | School bullying |
| 9 | Gastroesophageal reflux | |
| 10 | ||
| 11 | Gastroesophageal reflux | |
| 12 | H. pylori infection |
There was no evidence of neurologic, psychiatric and/or intellectual problems in any of the patients, and a stressor that may have triggered the syndrome was only identified in 5, although a causal association could not be established objectively (Table 5).
DiscussionRumination syndrome is a functional gastrointestinal disorder whose prevalence is largely unknown. It may have onset at any age, although certain age groups, such as adolescents, appear to be at higher risk. There is also a higher risk in the female sex.1–4 Interestingly, the demographic characteristics of our patients diverged from those described in the previous literature as regards both sex and age, with a predominance of the male sex and onset during childhood in most patients.
This is a complex disorder whose pathophysiology is not yet well understood and where physiological, sensory and/or psychological factors contribute to the onset and persistence of symptoms, potentially causing significant impairment. One of the most widely accepted pathophysiological theories proposes that rumination results from a voluntary and learned relaxation of the diaphragm which, combined with conscious but involuntary contractions of abdominal muscles, generates an increase in postprandial gastric pressure that overcomes the resistance of the lower oesophageal sphincter to the retrograde flow of contents.1–5
A specific trigger, such as an intercurrent disease or psychosocial stressor, may often precede the onset of rumination symptoms, something that occurs in other functional gastrointestinal disorders. Infections or stress seem capable of altering the gastrointestinal tract, making it more vulnerable to hyperalgesia and dysfunction in the long term. Currently, the general profile of patients with rumination syndrome is not well understood, and there is a broad variability in the psychological presentation of these patients. It is not clear whether psychological features are directly involved in the development of the disorder, or result from the underlying disease.1–4
None of the patients in our study had any kind of neurologic, psychological or intellectual problems, but stressor that may have triggered the syndrome was identified in 5. In two of them, rumination symptoms appeared following an episode of acute gastroenteritis, in one, after starting high school, in one following a period of being bullied in school, and in the last one after starting practicing a sport.
Due to the rarity of the disease and a limited knowledge of its clinical presentation on the part of clinicians, patients are misdiagnosed and are often subjected to tests and treatments that are unnecessary, invasive and costly.2,3 Delays in diagnosis and treatment may lead to the development of complications, such as weight loss, malnutrition, electrolyte imbalance and functional impairment. There are also emotional consequences of incorrect diagnosis, such as distress and anxiety.2,3
The diagnosis of rumination syndrome is based on the Rome IV criteria.1 As occurs in most other functional gastrointestinal disorders, rumination is a diagnosis of exclusion in which a thorough history taking is essential, as it may be the only means required to diagnose this disorder, without need for specific diagnostic tests.2,4 The evaluation must focus on the identification of potential underlying infection, malignancy, metabolic and/or structural disorder before diagnosing rumination syndrome. Furthermore, rumination needs to be differentiated from other gastrointestinal diseases that also present with regurgitation or vomiting as the main symptom, such as GERD, achalasia, gastroparesis and/or cyclic vomiting syndrome.1,2,4
The importance of direct observation of rumination episodes while the patient eats should not be disregarded. However, in cases where the diagnosis is still uncertain, there are two tests that can provide objective data on rumination episodes and support the diagnosis.2,5
Antroduodenal manometry consists in the measurement of intraluminal changes through a catheter inserted orally through the stomach and small intestine, and is one of the techniques that may be of use in the diagnosis of rumination. The resulting manometric pattern is characterised by a sudden and brief increase in pressure (peaks termed R waves) generated by the contraction of abdominal wall muscles and preceding the retrograde flow of gastric contents into the oesophagus.2–5
Multichannel intraluminal impedance associated to high-resolution manometry in the postprandial period can detect increases in abdominal pressure (manometry) immediately preceding the movement of a retrograde bolus into the oesophagus (impedance) during rumination episodes.4,5
The most salient finding as regards the diagnostic tests performed in the cases under study is that every single patient was subjected to at least one test that was not needed for the diagnosis of rumination, while only 3 patients were eventually assessed by antroduodenal manometry after performance of other tests that were unnecessary.
It is also worth reiterating that some of our patients, as described in the previous literature, had the same diagnostic test done on several occasions despite obtaining normal results, so that they were subjected to multiple avoidable tests.
When it comes to other diagnoses, most of our patients received diagnoses of other disorders before the diagnosis of rumination syndrome, such as GERD, H. pylori infection, eosinophilic oseophagitis, abdominal migraine and even superior mesenteric artery syndrome.
Pharmacological treatment is of little use in rumination syndrome. Most patients are initially treated with drugs that suppress acid production for possible GER, without symptom improvement.2 Other commonly used drugs are prokinetic agents, which are also ineffective.2 There is recent evidence that baclofen may be useful for treatment of rumination syndrome. Baclofen is a gamma-aminobutyric acid (GABAB) receptor agonist and has proven useful in increasing basal lower oesophageal sphincter pressure and inhibiting the sphincter's transient relaxations, thus reducing the acid and non-acid reflux events in patients with GERD; an increased lower oesophageal sphincter pressure may be useful in patients with rumination, as it would increase the pressure that needs to be overcome for gastric contents to flow back to the mouth. The dosage in adults is of 10mg before each meal.6,7
The treatment of rumination syndrome aims to modify the underlying mechanism that causes it, that is, the voluntary contraction of the abdominal wall, by abdominal retraining techniques. Biofeedback is a widely used method in medicine. A feedback system provides the patient with information on a physiological activity that the patient is performing incorrectly unconsciously but voluntarily, so that the patient can correct it consciously and voluntarily. The use of biofeedback for treatment of rumination consists in monitoring abdominothoracic muscular activity by means of electromyography and training the patient to relax abdominal and intercostal muscles to prevent further episodes in the course of 3 sessions.5,6
At present, research is being conducted on the experimental use of electromyography-guided control of abdominal muscles in patients with rumination. This biofeedback method can help develop voluntary control of the contraction of the muscles involved in rumination episodes through diaphragmatic breathing exercises. The results obtained to date in children as well as adults have been encouraging.5,6
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Giménez Casado A, López Liñán MJ, Barba Orozco E, Accarino Garaventa A, Álvarez Beltrán M, Azpiroz Vidaur F, et al. Rumination syndrome: Diagnostic and therapeutic difficulties of a not so uncommon disorder. An Pediatr (Barc). 2018;88:100–105.
Previous presentation: This study was presented at the XXIII Congress of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica; May 12–14, 2016; Gijón, Spain.