Streptococcus pyogenes or Group A Streptococci (GAS) cause many infections in infancy. Changes in its epidemiology have been described in recent years, including an increase in invasive infections (iGAS).
MethodsA retrospective-descriptive study was conducted on children less than 15-years-old, with GAS infections, in particular iGAS, and their complications from February 2004 to April 2014.
ResultsA total of 2192 positive cultures were obtained of which 92.7% were pharyngeal cultures. Twenty-nine patients were admitted to hospital: 4 with suppurative complications, 7 post-infective, 14 iGAS, and 4 probable iGAS cases. There were no differences in the frequency of GAS isolations/year. Non-invasive isolates were more frequent in winter and spring (P<.001), and 68.3% were in patients younger than 5 years.
The incidence of iGAS was 2.1/100000 children/year. There was no seasonality, and it was more frequent in younger children (P=.039). The most common diagnosis was pneumonia (6/14). Eight patients required intensive care. They were treated empirically with second or third-generation cephalosporin or with intravenous penicillin, and pneumonia required longer treatment times (P=.016). All GAS isolates were sensitive to penicillin, and 10.6% were resistant to erythromycin. The time spent in hospital was longer for iGAS than other cases (P=.028). No patients died.
ConclusionsPharyngotonsillitis caused by GAS is common in childhood, and its incidence is increasing in children younger than 5 years. At the moment, post-infectious complications are rare. Invasive infections are the most severe forms of presentation, and are more common in younger children.
Streptococcus pyogenes o estreptococo del grupo A (EGA) causa numerosas infecciones en la infancia. En los últimos años se han descrito cambios en su epidemiología, con un aumento de las infecciones invasivas.
MétodosEstudio retrospectivo-descriptivo en menores de 15 años con infección por EGA y sus complicaciones, desde febrero de 2004 a abril de 2014.
ResultadosSe obtuvieron 2.192 cultivos positivos, siendo el 92,7% faringoamigdalares. Ingresaron 29 pacientes: 4 complicaciones supurativas, 7 postinfecciosas, 14 infecciones invasivas y 4 probables. No hubo diferencias en la frecuencia de aislamientos de EGA/año. Los aislamientos no invasivos fueron más frecuentes en invierno y primavera (p<0,001), siendo el 68,3% de los pacientes menores de 5 años.
La incidencia de infecciones invasivas fue de 2,1/100.000 niños/año. No mostraron estacionalidad y ocurrieron en niños de menor edad (3,3±2,2 vs. 4,9±2,9 años, p=0,039). El diagnóstico más frecuente fue la neumonía (6/14) y el lugar de aislamiento fue la sangre (8/14). Ocho precisaron cuidados intensivos. Se trataron empíricamente con cefalosporinas de segunda/tercera generación o penicilina intravenosas. Las neumonías precisaron mayor tiempo de tratamiento que el resto (13,8±3,5 vs. 11±2 días, p=0,0016). Todos los EGA fueron sensibles a penicilina, el 10,6% resistentes a eritromicina. El tiempo de ingreso fue mayor en las infecciones invasivas (13±5 vs. 8,7±4,4 días, p=0,028). Ningún paciente falleció.
ConclusionesLa faringoamigdalitis por EGA sigue siendo frecuente en la infancia y su incidencia está aumentando en menores de 5 años. En la actualidad, las complicaciones postinfecciosas son raras. Las infecciones invasivas son las formas de presentación más grave y son más frecuentes en niños de menor edad.
Group A β-haemolytic streptococcus (GAS), or Streptococcus pyogenes, is one of the most frequent pathogens in the paediatric age group. It produces disease of varying severity, from acute pharyngitis (AP) and its suppurative complications to forms associated with a high mortality, including post-infection complications (rheumatic fever [RF] and post-streptococcal glomerulonephritis [PSGN]) and invasive disease.1,2
In the past few decades, there has been a generalised increase in the incidence of invasive disease in Europe and North America, the cause of which has yet to be determined.3 In Europe, the incidence is of 2.79 cases per 100000 inhabitants per year,3 with an estimated incidence in the paediatric population of 0.12–3.1 per 100000 children per year.4–7 The mortality in children ranges from 3.6% to 8.3%,8 but it can reach 26.8% for its most severe form, streptococcal toxic shock syndrome (STSS).9
The main risk factors for invasive disease are extreme age (age<5 years or >65 years), immunosuppressed states, varicella, diabetes, and skin lesions.3,5,9–12 Its most frequent presentations are occult bacteraemia4,5 or cellulitis,3,7 and GAS is most frequently isolated from blood samples.4,7
The studies on invasive disease conducted in Europe mainly involved adult patients,7,12 and the few paediatric case series published in Spain focused on STSS.9 The aim of our study was to assess the frequency of infection by GAS, its complications and especially invasive disease in the paediatric population served by our hospital.
Materials and methodsWe conducted a retrospective descriptive study of children aged less than 15 years with infection by GAS managed in the Department of Paediatrics of the Hospital Clínico Universitario of Valencia (Spain) between February 2004 and April 2014. This is a tertiary referral hospital with 79 paediatric beds that manages an average of 25522 emergency visits and 2748 paediatric admissions per year. Its catchment area includes 52735 children, although the paediatric intensive care unit (PICU) receives patients from other health areas. The study was approved by the ethics committee of the hospital.
Cultures that were positive for GAS were recorded in the Department of Microbiology database, including those with isolation of non-invasive strains from samples obtained in primary care facilities and submitted to our hospital. The decision to order culture or rapid antigen detection testing (RADT) for the diagnosis of AP was made on a case-by-case basis. We also reviewed the health records of patients admitted to hospital.
We defined suppurative complications as the spread of GAS infection to structures adjacent to the pharynx.13 Rheumatic fever was diagnosed according to the Jones criteria14 and PSGN based on the presence of clinically compatible illness with culture from throat or skin specimen positive for GAS or elevation of serum streptococcal enzymes and a transient reduction in the levels of complement component C3.15 We defined confirmed invasive infection as isolation of GAS from a normally sterile site,16 and probable invasive infection as clinical evidence of invasive disease in the absence of another identified aetiology with isolation of GAS from a nonsterile site.17 The diagnosis of STSS was made according to the criteria of the Working Group on Severe Streptococcal Infections.16
We collected data on the following:
- 1.
For patients with positive isolates: age, sex, season, site of isolation and results of antibiotic susceptibility testing. Due to changes to the database of the Department of Microbiology, we only had data on the age and RADT for patients with non-invasive isolates for the last 3 years.
- 2.
For hospitalised patients (with complications or invasive disease): in addition to all of the above, personal medical history (risk factors, previous medical treatment, other), clinical manifestations, main laboratory results (complete blood count, C-reactive protein [CRP] and procalcitonin [PCT]), treatments given, outcome, length of stay, and mortality scores (Pediatric Index of Mortality [PIM2] and Pediatric Logistic Organ Dysfunction [PELOD]) for patients admitted to the PICU.
Microbiological tests were performed at the Department of Microbiology of the hospital. Clinical specimens were processed following standard protocol. Throat swab specimens were directly plated on CNA agar and inoculated in Todd–Hewitt broth (BD Diagnostics), which were incubated at 37°C for 24–48h. Todd–Hewitt broth subcultures on CNA plates were performed when the primary cultures were negative. Group A streptococcus was identified based on phenotypic characteristics (catalase-negative, β-haemolytic activity and susceptibility to bacitracin [0.04U]).18 Antimicrobial susceptibility was assessed by means of disc diffusion tests following the standards of the Clinical and Laboratory Standards Institute (CLSI).19
We performed the statistical analysis with the statistical software SPSS version 15.0. We set the level of statistical significance at 0.05. We selected statistical tests that would maximise power. If any of the assumptions for a statistical test were not met, we used a robust alternative (such as the Welch test). We analysed continuous dependent variables (such as age or length of stay) by fixed-effects ANOVA, ascertaining the homogeneity of variance by means of the Levenne test. We also calculated the effect size (partial η2) and the power of the test. For qualitative variables (such as season of the year or hospital admission) we used the χ2 test or, if its conditions were not met, the Monte Carlo simulation method.
ResultsThere were 2192 cultures positive for GAS: 14 isolates from sterile sites and 2178 non-invasive isolates (2030 from pharynx/tonsils; 55 from the ear; 28 from skin, 20 from sputum and 45 from exudates at other sites). Twenty-nine patients were admitted to hospital: 4 with suppurative complications, 7 with post-streptococcal complications (PSGN in 5 and RF in 2), 14 with invasive disease and 4 with probable invasive disease.
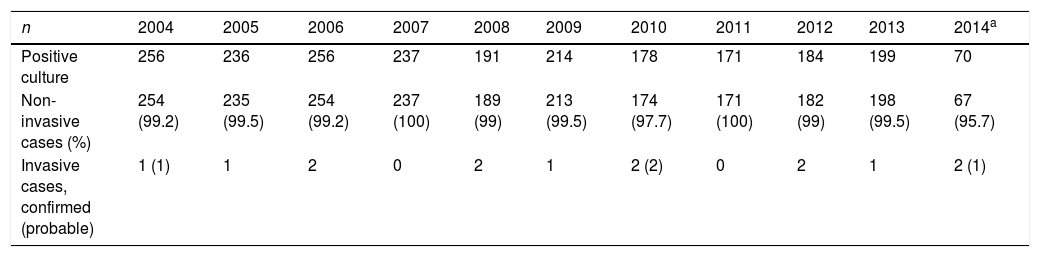
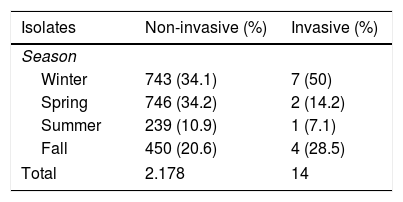
We found no differences in the annual incidence of non-invasive isolates throughout the study (Table 1), although the incidence did increase significantly in winter and spring (P<.01) (Table 2). There were no differences in incidence between sexes. We were only able to calculate the mean age for the patients in the last 3 years (678 patients with positive cultures), which was 4.9±2.9 years (median, 4 years); 68.3% of these patients were aged less than 5 years. Rapid antigen detection tests performed during this period had a sensitivity of 71.5% and a specificity of 96.1%.
Distribution of positive cultures, invasive cases and non-invasive cases in the years under study.
| n | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive culture | 256 | 236 | 256 | 237 | 191 | 214 | 178 | 171 | 184 | 199 | 70 |
| Non-invasive cases (%) | 254 (99.2) | 235 (99.5) | 254 (99.2) | 237 (100) | 189 (99) | 213 (99.5) | 174 (97.7) | 171 (100) | 182 (99) | 198 (99.5) | 67 (95.7) |
| Invasive cases, confirmed (probable) | 1 (1) | 1 | 2 | 0 | 2 | 1 | 2 (2) | 0 | 2 | 1 | 2 (1) |
When it came to patients admitted to hospital (with complications or invasive disease), we did not find significant differences in the annual incidence throughout the study or a seasonal pattern of infection (Tables 1 and 2).
In the last 2 years, RF was diagnosed in 2 girls aged 11 and 9 years. The first one presented with fever and a subcutaneous nodule in the neck, small joint pain and elevated acute phase reactants (erythrocyte sedimentation rate [ESR], 87mm/first hour), with no clear history of AP, but with an elevated antistreptolysin O (ASO) titre (882IU/mL). The second one sought care 15 days after having been treated correctly for AP, with a scarlatiniform rash but without isolation of GAS from pharyngeal exudate. She presented with fever, polyarthritis, joint pain and elevation of acute phase reactants (ESR, 71mm/first hour; CRP, 218mg/L) and of ASO (2105IU/mL). Both patients were treated with acetylsalicylic acid. They did not have cardiovascular complications. At the time of this writing, they remain asymptomatic and continue to receive penicillin prophylaxis.
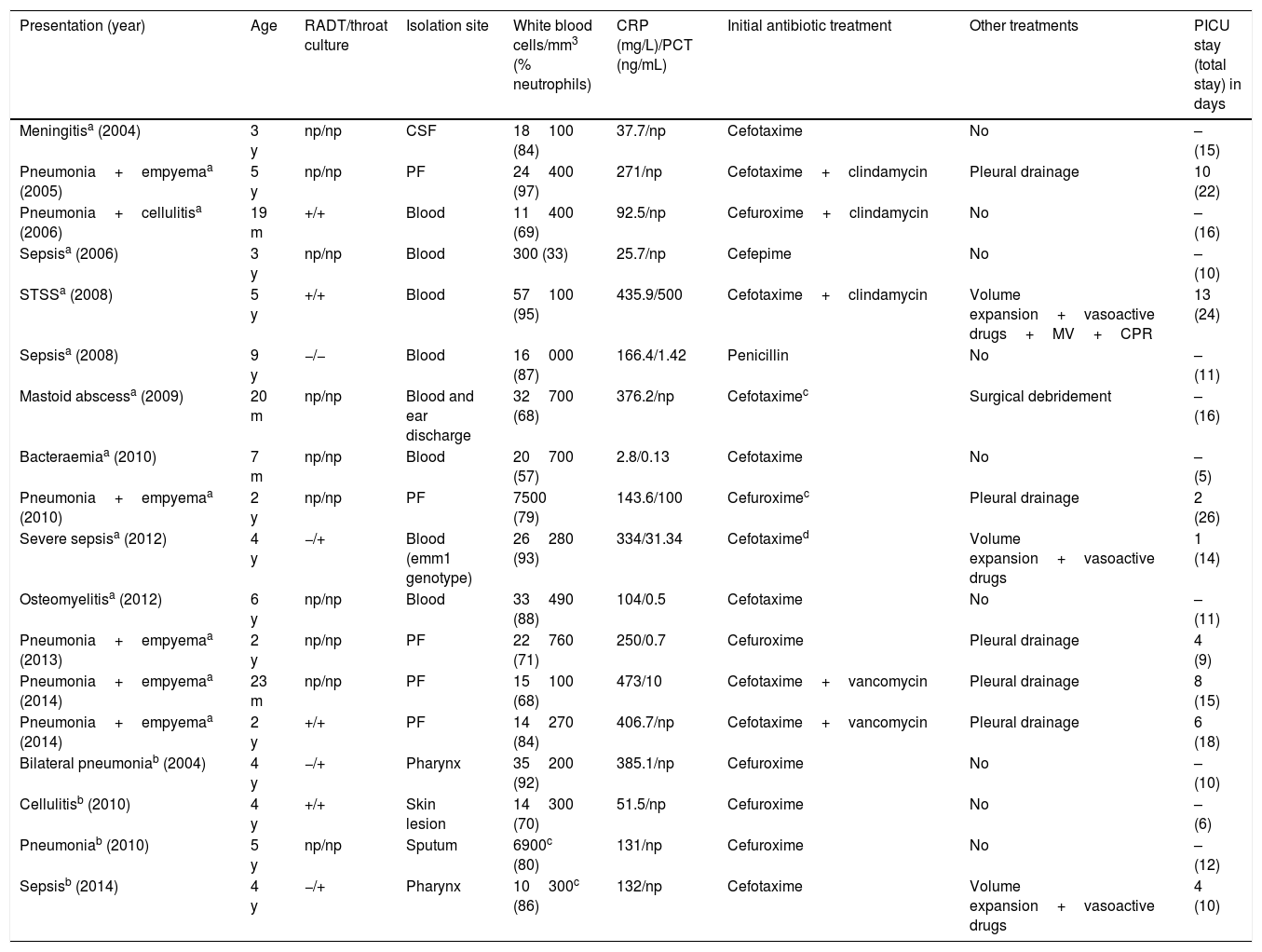
As for invasive infection, the incidence in our area (11 of the 14 cases) was 2.1 per 100000 children per year (0.5% of GAS isolates). The mean age was 3.3±2.2 years. Eleven patients had risk factors: 10 were aged less than 5 years, 2 had varicella, 1 had post-chemotherapy aplasia, and 1 was receiving corticosteroid treatment. Five patients had a previous history of disease of probable viral aetiology. A potential portal of GAS entry was identified in 11 patients: pharyngitis in 8, skin lesions in 2 and suppurative otitis media in 1. The most frequent diagnosis in cases of invasive infection was pneumonia with empyema (6/14) (Table 3). Group A streptococcus was isolated most frequently from blood (8/14). Genotyping was only performed in 1 patient (emm1).
Data on confirmed and probable cases of invasive disease.
| Presentation (year) | Age | RADT/throat culture | Isolation site | White blood cells/mm3 (% neutrophils) | CRP (mg/L)/PCT (ng/mL) | Initial antibiotic treatment | Other treatments | PICU stay (total stay) in days |
|---|---|---|---|---|---|---|---|---|
| Meningitisa (2004) | 3 y | np/np | CSF | 18100 (84) | 37.7/np | Cefotaxime | No | – (15) |
| Pneumonia+empyemaa (2005) | 5 y | np/np | PF | 24400 (97) | 271/np | Cefotaxime+clindamycin | Pleural drainage | 10 (22) |
| Pneumonia+cellulitisa (2006) | 19 m | +/+ | Blood | 11400 (69) | 92.5/np | Cefuroxime+clindamycin | No | – (16) |
| Sepsisa (2006) | 3 y | np/np | Blood | 300 (33) | 25.7/np | Cefepime | No | – (10) |
| STSSa (2008) | 5 y | +/+ | Blood | 57100 (95) | 435.9/500 | Cefotaxime+clindamycin | Volume expansion+vasoactive drugs+MV+CPR | 13 (24) |
| Sepsisa (2008) | 9 y | −/− | Blood | 16000 (87) | 166.4/1.42 | Penicillin | No | – (11) |
| Mastoid abscessa (2009) | 20 m | np/np | Blood and ear discharge | 32700 (68) | 376.2/np | Cefotaximec | Surgical debridement | – (16) |
| Bacteraemiaa (2010) | 7 m | np/np | Blood | 20700 (57) | 2.8/0.13 | Cefotaxime | No | – (5) |
| Pneumonia+empyemaa (2010) | 2 y | np/np | PF | 7500 (79) | 143.6/100 | Cefuroximec | Pleural drainage | 2 (26) |
| Severe sepsisa (2012) | 4 y | −/+ | Blood (emm1 genotype) | 26280 (93) | 334/31.34 | Cefotaximed | Volume expansion+vasoactive drugs | 1 (14) |
| Osteomyelitisa (2012) | 6 y | np/np | Blood | 33490 (88) | 104/0.5 | Cefotaxime | No | – (11) |
| Pneumonia+empyemaa (2013) | 2 y | np/np | PF | 22760 (71) | 250/0.7 | Cefuroxime | Pleural drainage | 4 (9) |
| Pneumonia+empyemaa (2014) | 23 m | np/np | PF | 15100 (68) | 473/10 | Cefotaxime+vancomycin | Pleural drainage | 8 (15) |
| Pneumonia+empyemaa (2014) | 2 y | +/+ | PF | 14270 (84) | 406.7/np | Cefotaxime+vancomycin | Pleural drainage | 6 (18) |
| Bilateral pneumoniab (2004) | 4 y | −/+ | Pharynx | 35200 (92) | 385.1/np | Cefuroxime | No | – (10) |
| Cellulitisb (2010) | 4 y | +/+ | Skin lesion | 14300 (70) | 51.5/np | Cefuroxime | No | – (6) |
| Pneumoniab (2010) | 5 y | np/np | Sputum | 6900c (80) | 131/np | Cefuroxime | No | – (12) |
| Sepsisb (2014) | 4 y | −/+ | Pharynx | 10300c (86) | 132/np | Cefotaxime | Volume expansion+vasoactive drugs | 4 (10) |
−, negative; +, positive; CPR, cardiopulmonary resuscitation; CRP, C-reactive protein; CSF, cerebrospinal fluid; m, months; MV, mechanical ventilation; np, not performed; PCT, procalcitonin; PF, pleural fluid; PICU, paediatric intensive care unit; RADT, rapid antigen detection test; STSS, streptococcal toxic shock syndrome; y, years.
All patients presented with high fever (39.2±0.49°C) and 3 with skin rash: one with generalised erythema, one with scarlatiniform rash and one with nonspecific rash. At the time of admission, all patients had leukocytosis (except the patient with aplasia) with neutrophilia (23062±12817 white blood cells/mm3 with 80±12% neutrophils) and elevated acute-phase reactants (CRP, 239.76±155.70mg/L; PCT, 92±183.41ng/mL) (Table 3). The levels of CRP and PCT were normal in one patient with fever of 3hours’ duration.
Of all patients with probable invasive disease (Table 3), 2 had been given antibiotics 24h before admission, and 1 had had varicella one week earlier. All presented with high fever and elevated CRP.
Eight patients required admission to the PICU (Table 3). The mortality scores in the first 24h were: PIM2, 13.2±23.9% (range, 0.8–49.1%) and PELOD, 6.9±12.8% (range, 0–26.1%). The mean length of stay in the PICU was 6±4.1 days.
All patients with invasive infections received empirical treatment with second- or third-generation cephalosporins or penicillin. Nine patients required other treatments (Table 3). One patient developed a lung abscess that did not require surgical intervention. Three developed disseminated intravascular coagulation and acute renal failure. None of the patients died. The mean length of stay was 14±5.1 days.
All GAS isolates in the study (non-invasive and invasive) were sensitive to penicillin, cephalosporins and clindamycin, and we found a 10.6% prevalence of erythromycin resistance.
We compared different groups of patients, and found the following:
- -
The age of patients with invasive disease was significantly lower compared to patients with non-invasive disease (3.3±2.2 vs. 4.9±2.9 years; P=.039);
- -
The mean length of stay of patients with invasive disease was significantly longer compared to patients admitted for complications (14±5.1 vs. 8.9±3.9 days; P=.006);
- -
The duration of intravenous antibiotherapy was significantly greater in patients with pneumonia compared to all other patients (13.8±3.5 vs. 11.1±2 days; P=.016).
Although the early use of antibiotics and improvements in health care have reduced the incidence of complications, infection by GAS continues to be a significant public health problem, especially in developing countries.1,20,21 Acute pharyngitis is the most frequent presentation.22 In our study, GAS was isolated from pharyngeal specimens in more than 90% of cases, and its incidence did not change during the period under study.
Although the ear is not generally considered a frequent site of infection by GAS, it was the second most frequent site of isolation of S. pyogenes in our study. Taking into account that culture of ear discharge specimens was only performed in cases of suppurative otitis media, it would be fair to assume that the incidence of streptococcal otitis media may be higher than reported in the literature (3–5%).23
In the last 3 years of the study, more than two thirds of positive throat cultures occurred in children aged less than 5 years, which suggests an epidemiological shift that could be due to earlier enrolment of infants and young children in childcare and early childhood education centres.
In regards to complications, we ought to highlight the recent diagnosis of 2 cases of RF. Although they both occurred in the last 2 years, we cannot infer anything from it; nevertheless, they may warrant a separate analysis, since RF is practically nonexistent in developed countries.
There is evidence in the literature of an increase in the incidence of invasive infections since the 1980s.3 Still, the incidence continues to be very low, with invasive infection fulfilling the criteria for the definition of a rare disease established by the Health and Food Safety directorate of the European Commision.24 Although infection by GAS is frequent in Spain, its incidence was low in our case series and did not increase during the period under study. Contrary to previous studies,3 we did not find a seasonal pattern, which may have been due to the small sample size or the temperate climate of the geographical region where the study was conducted.
It is worth noting that cases of invasive disease predominantly occurred in preschool-aged children, in who diagnosis of streptococcal infection is more challenging.
Although a portal of entry may not be identified in approximately half of cases,4,25 we were able to identify a potential portal in nearly 80% of our patients (11/14), with the pharynx being the most frequent in our sample, in contrast with previous studies,12,26 where skin lesions were the most frequent portal of entry.7,12 For this reason, we would recommend performance of throat culture in all cases of suspected severe bacterial infection of unknown aetiology presenting with pharyngeal hyperaemia, however mild, as it could guide the aetiological diagnosis and even detect a potential invasive infection.
Varicella is considered the main risk factor in children,10,12 although in our study, only 2 cases were associated with this disease. This may be due to the lower incidence of varicella in the period under study due to routine vaccination of the population (2006–2012 incidence, 389 cases/100000 inhabitants/year).27
Contrary to other series,3,7 pneumonia was the most frequent presentation in our series and another recent study.28 We ought to note that half of the cases of pneumonia occurred in the last year of the study, which alerts us to the possibility of an increase in its incidence. Furthermore, this presentation is usually associated with prolonged hospitalisation,29 as was the case in our study. We found no cases of necrotising fasciitis, which is rare in children.3,30
Severe bacterial infection is relatively easy to diagnose: fever with leukocytosis and neutrophilia and/or elevation of acute phase proteins. On the other hand, the aetiological suspicion of GAS is harder to establish, unless the patient presents with the characteristic rash.
At any rate, empirical treatment was effective in every case, since, consistent with the previous literature,5,7,12 all isolates were sensitive to penicillin and cephalosporins. Once GAS is identified, empirical treatment can be switched to penicillin. Clindamycin was added to the regimen in the most severe cases. Although clindamycin resistance did not occur in the sample under study, its prevalence can be as high as 6.8%31 in Spain, so it should never be used as monotherapy.12,32 There were, however, cases of erythromycin resistance, which have increased in frequency in recent decades, especially in Spain, where the prevalence of resistance to 14- and 15-membered macrolides ranges between 10% and 41%.7,12,33 Therefore, it is recommended that macrolides be used only in patients allergic to beta-lactam antibiotics (preferably 16-membered macrolides).
Nearly half of the patients with invasive disease required admission to the PICU (Table 3). The most severe presentation was STSS (PELOD score of 26.1% and PIM2 score of 49.1%), but its outcomes were good. Intravenous immunoglobulin therapy was not used because there is no evidence that it reduces mortality or lengths of stay in these patients.30
Vaccination may be the only possible means to control and eradicate GAS infection, but no vaccines are commercially available at present.8
ConclusionsS. pyogenes is a frequent cause of infectious disease in children, especially in winter and spring. Our study found a higher incidence in younger patients (age<5 years). Although its complications are currently rare, their potential severity underscores the importance of an early and accurate diagnosis of group A streptococcal pharyngitis and adherence to prescribed antibiotic treatment.
Invasive disease constitutes the most severe presentation of GAS infection and occurs more frequently in early childhood, when the diagnosis of streptococcal infection poses a greater challenge. We did not find changes in incidence in the past 10 years. The clinical presentation of GAS infections is variable and frequently nonspecific, but the diagnosis of severe bacterial infection should not be difficult, and empirical antibiotherapy is effective. Early diagnosis and treatment are crucial on account of the potential severity of disease, which may require intensive supportive care in some cases.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Espadas-Maciá D, Flor Macián EM, Borrás R, Poujois Gisbert S, Muñoz Bonet JI. Infección por estreptococo pyogenes en la edad pediátrica: desde faringoamigdalitis aguda a infecciones invasivas. An Pediatr (Barc). 2018;88:75–81.