Infantile haemangiomas (IHs) are the most frequent type of benign tumours in children; however, they may be problematic, leading to complications such as ulceration, functional impairment, hypothyroidism or disfigurement, in which case propranolol is the first-line treatment. In addition, IHs, depending on the location, may be associated to other diseases such as PHACE syndrome, LUMBAR syndrome or, if there are 5 or more cutaneous lesions, liver haemangioma. Screening ultrasonography is recommended to rule the latter out.1
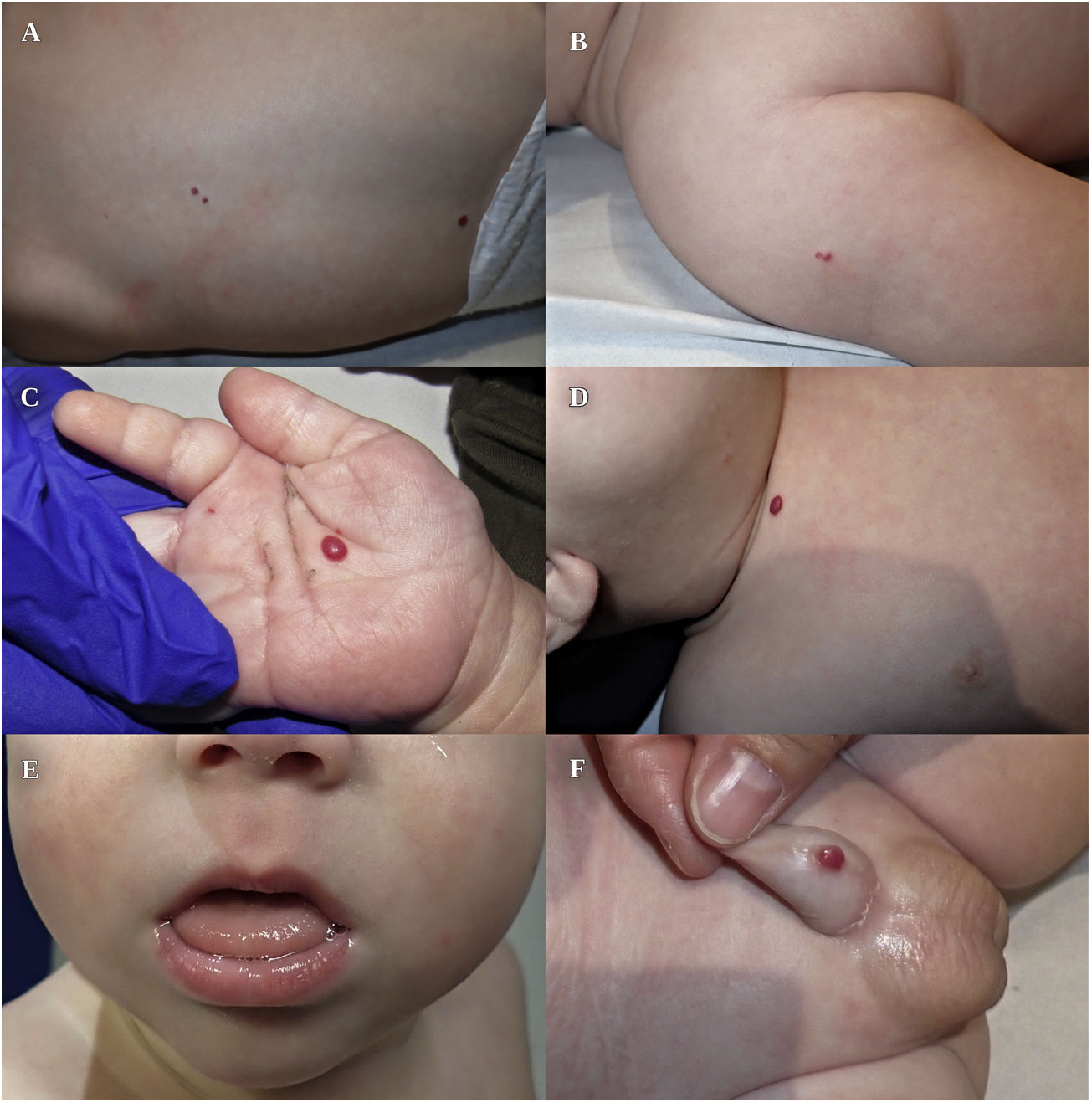
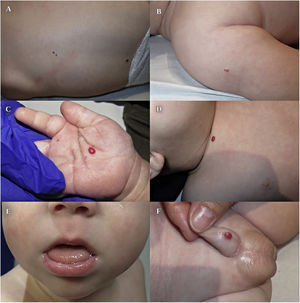
We present the case of an infant aged 3 months with asymptomatic cutaneous lesions with a vascular appearance present from birth. The salient personal history included conception with assisted reproductive technology (ART), delivery by caesarean section due to suspected subclinical chorioamnionitis (week 28+2), large weight and length for gestational age (at the 92th and 98th percentiles), umbilical hernia, mild macroglossia and hyaline membrane disease treated with one dose of surfactant. The family history was unremarkable. The physical examination revealed 7 purplish-red papules with a vascular appearance, 2–6 mm in diameter, located in the right palm, penis, back, clavicle and right shoulder, compatible with multiple IHs (Fig. 1).
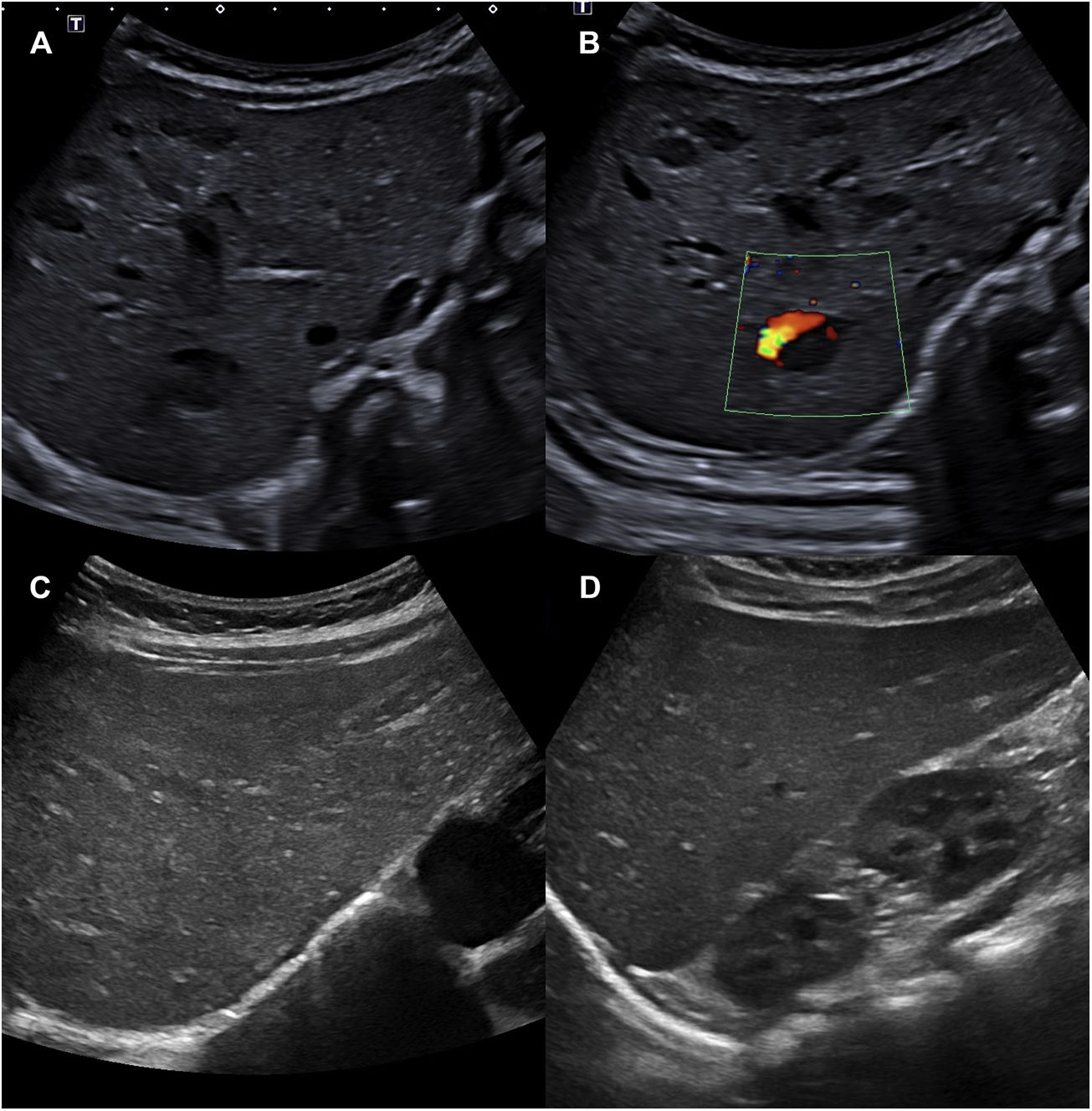
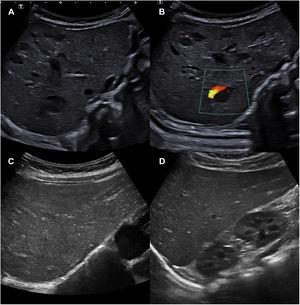
Due to the presence of more than 5 haemangiomas, an ultrasound examination of the abdomen was performed, revealing between 10 and 15 space-occupying lesions, hypoechogenic, oval, some with a Doppler signal within, compatible with hepatic haemangioma (Fig. 2A and B). The complete blood count, thyroid and liver function panels and the alpha-foetoprotein test did not detect any relevant abnormalities. Early treatment was initiated with oral propranolol at a dose of 1 mg/kg every 12 h for a duration of 1 year, with resolution of the hepatic haemangiomas within 4 months (Fig. 2C and D) and progressive regression of the cutaneous haemangiomas. Given the presence of macroglossia, umbilical hernia and macrosomia, the patient exhibited 1 cardinal feature and 2 suggestive features (4 points in total) for the diagnosis of Beckwith-Wiedemann syndrome (BWS). When a patient has a score of 4 points or greater, it is possible to make a clinical diagnosis of BWS without awaiting the results of genetic testing, although testing is still recommended.2 Testing of the 11p15.5 locus by means of methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) evinced loss of methylation of the imprinting control region 2 (ICR2), compatible with BWS.
A and B) Abdominal ultrasound scan with visualization of 10 to 15 hypoechogenic space-occupying lesions compatible with hepatic haemangioma. A Doppler signal can be seen in image B. C and D) Abdominal ultrasound scan at 4 months of treatment, in which the hepatic haemangiomas can no longer be seen.
Beckwith-Wiedemann syndrome is the most frequent overgrowth syndrome, with a prevalence of 1 case per 10 340 naturally conceived births and 1 case per 1126 births from ART.3 It is characterised by macroglossia, macrosomia, abdominal wall defects, neonatal hypoglycaemia and an increased risk of embryonal tumours.2–6 However, vascular tumours are rare in affected patients.
This syndrome is most frequently caused by genomic imprinting defects (epigenetic disorders) in chromosome region 11p15.5 in 2 critical domains: imprinting control regions 1 and 2 (ICR1 and ICR2). The most frequent causative defect (50% of cases) is loss of methylation in domain ICR2 in the maternal allele, which results in inhibition of the expression of CDKN1C, a cell cycle inhibitor gene. Less frequently, BWS may be secondary to uniparental disomy in the 11p15 region (20%) or gain of methylation at the maternal ICR1 allele (5%) leading to overexpression of the insulin-like growth factor 2 (IGF2) gene and repression of the non-translated long non-coding RNA H19 tumour-suppressor gene. These changes give rise to the overgrowth phenotypes associated with this syndrome.2–4
Infantile haemangiomas occur in approximately 4% to 5% of births, and they are the most common type of tumour in infants.1 Their pathogenesis is not well understood, although recent studies have found increased expression of IGF2 in the proliferative phase of IH, suggesting that this protein may play a key regulator of haemangioma proliferation.7 The association of IH and BWS may involve the IGF27 and CDKN1C genes, as both are strong regulators of foetal growth. In the case of our patient, the identified molecular defect was loss of methylation of ICR2, resulting in inhibition of the expression of CDKN1C and therefore in cellular proliferation.
There are few reports in the literature of cases of association of BWS with IH. Recently, Macchiaiolo et al described one patient in whom BWS (paternal uniparental disomy) was associated with cutaneous and hepatic haemangiomas.2,5 Previously, Francisco et al had published a case of BWS associated with multiple IHs in the lung, liver and axilla.6 Isolated IHs in the spleen, liver or even the placenta have also been described in patients with SBW.2 To our knowledge, this is the second instance of BWS associated with multiple cutaneous and hepatic IHs reported in the literature.
In conclusion, vascular tumours, and multiple IHs in particular, may be associated with BWS and be part of its disease spectrum, so they should be taken into account in the differential diagnosis to allow early diagnosis and treatment.