Vaccines against rotavirus (RV) have been available in Spain since 2006, but they are neither recommended nor financed by the National Health System. Nevertheless, through recommendations of the Spanish Association of Paediatrics vaccination has achieved intermediate coverage.
Material and methodsA systematic literature review was performed on studies carried out in Spain in the last 12 years (2006–2018) on RV infection and vaccination.
ResultsA total of 43 studies were identified that met the inclusion criteria. The disease burden in children less than 5 years in the Primary Care setting ranged from 15 to 19 cases per 1000 children, and between 120 and 480 cases per 100,000 in the hospital setting, which has a significant economic and social impact. Vaccines against RV have shown an effectiveness of between 83% and 96%, and an impact of up to 70% in reducing hospital admissions, which is dependent on the achieved vaccine coverage. New research lines are identified, such as the role of the rotavirus vaccine and protection against seizures or the impact on the gut microbiota.
ConclusionsThe current available information supports the significant burden of rotavirus disease in Spain and the high effectiveness of the available vaccines. This evidence should allow for an updated re-evaluation of the national recommendations on rotavirus vaccination.
En España, las vacunas frente a rotavirus (RV) están disponibles desde 2006 pero no están ni recomendadas ni financiadas por el Sistema Nacional de Salud. Sin embargo, a través de las recomendaciones de la Asociación Española de Pediatría se han alcanzado coberturas de vacunación intermedias.
Material y métodosSe ha realizado una revisión sistemática de la literatura sobre los estudios realizados en España en los últimos 12 años (2006-2018) en relación con la infección y las vacunas frente a RV.
ResultadosSe identifican 43 estudios que cumplían los criterios de selección. La carga de enfermedad en población <5 años en atención primaria oscila entre 15 y 19 casos por 1.000 niños y en hospitalaria entre 120 y 480 casos por 100.000, lo que supone una importante repercusión económica y social. Las vacunas frente a RV han mostrado en España una efectividad de entre el 83 y el 96% y un impacto de hasta un 70% de reducción de hospitalizaciones, que es dependiente de la cobertura de vacunación alcanzada. Se identifican además nuevas líneas de investigación relacionadas con el papel de la vacuna del RV y la protección frente a convulsiones, o el papel del microbiota, entre otros.
ConclusionesLa información actualmente disponible refrenda la importante carga de enfermedad por RV en España y la elevada efectividad de las vacunas disponibles. Estas evidencias permiten una reevaluación de las recomendaciones nacionales sobre vacunación frente a RV.
Rotavirus (RV) is the main cause of severe acute gastroenteritis in children worldwide. Improvements in hygiene and sanitation have a limited impact in the control of acute rotavirus gastroenteritis (RVGE), and vaccination is therefore the best strategy for preventing this disease. Based on estimates made by the World Health Organization (WHO), the global mortality associated to infection by this virus has decreased by more than 50% after the introduction of routine vaccination against RV in more than 50 countries (http://www.who.int/immunization/diseases/rotavirus/en/).
In Spain, vaccines against RV were authorized for marketing in 2006. At that time, the Interterritorial Council of the National Health System recommended against including these vaccines in the routine immunization schedule of Spain. However, the Asociación Española de Pediatría (Spanish Association of Pediatrics) recommends vaccination against RV, which at present is performed by means of prescriptions by paediatricians and has achieved intermediate vaccination coverage rates.1 In 2010, the Spanish Agency of Medicines suspended the marketing of both rotavirus vaccines—for 5 months in case of the pentavalent vaccine (June to November of 2010) and 6 years in case of the monovalent vaccine (from June 2010 to June 2016)—as a precaution that had no actual impact on safety on account of the detection of circovirus fragments in the vaccines.2
At the time the vaccines were first introduced, there were insufficient data about the burden of disease in Spain, and therefore the potential impact of vaccination could not be estimated accurately. Since then, numerous studies have been conducted to assess these issues in Spain. In this article, we present a review of the published studies conducted in Spain about RV and the 2 RV vaccines from the time the vaccines were first authorized for commercialization in our country.
Materials and methodsWe performed a systematic review of the literature by searching the PubMed and Embase databases for studies conducted in Spain that evaluated aspects related to the burden of disease and/or the outcomes of vaccination against RV. We restricted the search to articles published in Spanish or English from 2006 (the year the RV vaccine was first introduced) to the time the search was performed (January 2018). To do so, we used a combination of MeSH terms related to disease caused by RV, its epidemiology and the clinical, economic and social impact of RV infection, as well as terms related to vaccination (effectiveness, impact, safety, efficiency). We also combined these terms with additional terms to restrict hits to studies performed in Spain or including Spanish samples.
We excluded studies that were not epidemiological, were not conducted in Spain or not conducted in humans and publications other than original articles (editorials, reviews, letters, commentaries and communication abstracts).
We classified the selected studies by their objectives and summarized their results to analyze the epidemiology of RV and the current situation of vaccination against RV in Spain.
Results and discussionThe search yielded a total of 128 studies, of which 43 met the selection criteria. We proceed to summarize and discuss the most relevant results of these works.
Burden of disease caused by rotavirusRotavirus gastroenteritis managed at the primary care levelThe reported annual incidence of RVGE managed at the primary care level ranges from 15.4 to 19.5 cases per 1000 children under 5 years and 20 cases per 1000 children under 3 years (Table 1). This variability could be explained by differences between the studies in methodology and the period under study. It is believed that active efforts to detect cases are generally less likely to be affected by biases. The reported proportion of RVGE cases relative to the total cases of AGE also varies between studies.
Burden of disease due to rotavirus managed in primary care in Spain.
| Study | Geographical area(Period under study) | Study population | Methodology | Results |
|---|---|---|---|---|
| Gerstel, 20093 | Aragon(1998–2006) | General populationN=1044888 cases of diarrhoea;89% in<5 years | Retrospective, passive surveillance of cases of diarrhoea notified to the disease surveillance system of Aragon and reports to microbiology laboratories | RV+: 17.1% (90.2% of cases with confirmed detection of RV).Increase in the mean number of RV+ tests from 22 to 47/100000 inhabitants/year |
| Díez-Domingo, 20064 | Valencia(December 2003–November 2004) | Children<5 years.N=553 cases of AGE | Prospective in 13 paediatrics clinics.Active search of cases of RV in children visiting the clinic with AGE.Detection of RV in stool samples by EIA (Rotaclone) and genotyping by PCR | RV+: 15.0%.Annual incidence: 15.4 cases/1000 Children under 5 years (31 por 1000 children under 1 year)80% of cases between January and March |
| Díez-Domingo, 20115 | Six European countries, including Spain (Valencia)(November 2005–May 2007) | Children under 5 years.N=2088 cases of AGE in Spain | Prospective in the paediatrics clinics of 9 primary care centres.Active identification of cases of RV among all the patients seeking care for AGE.Detection of RV in stool samples by immunochromatography (RotaStrip), and confirmation and genotyping by PCR | RV+: 12.8%.Confirmed by PCR: 11.5%.Annual incidence of RV+ detected by PCR: 19.5 cases/1000 children/year (38.0 per 1000 children per year in infants aged<1 year) |
| Arístegui, 20166 | Catalonia, Basque Country and Andalusia(October–April 2014) | Children under 3 years.N=1087 cases of AGE | Prospective in 64 paediatrics clinics and 2 hospital emergency departments.Active identification of cases of RV among all the patients seeking care for AGE.Detection of RV in stool samples by immunochromatography (Vikia Rota-Adeno) | RV+: 33.9%.Incidence during the 5-month followup: 2.01cases/100 children (73% of cases in children aged 6–324 months) |
EIA, enzyme immunoassay; PCR, polymerase chain reaction; RV+, proportion of AGE cases that tested positive for rotavirus.
These differences may be explained by: (a) health care factors (patients seeking care for illness of varying severity), (b) the duration of follow-up (analysis of the entire year versus epidemic seasons), (c) the methods used for diagnosis, each with a different sensibility and sensitivity, with the gold standard being polymerase chain reaction (PCR), whose use is currently restricted to the context of research, and (d) the natural seasonal fluctuations in the circulation of RV.
A European study conducted before the introduction of the rotavirus vaccines found an incidence of RVGE managed at the primary care level of 1.45–4.18 per 100 children under 5 years, and the proportion of total cases of AGE with positive detection of RV by enzyme-linked immunoassay (ELISA) ranged from 25.5% to 41.3%, depending on the country.7
Since these data are based only on the cases of patients that sought medical care, the documented incidence must be lower than the actual incidence, as it is estimated that a significant proportion of affected children do not seek care from a paediatrician.8
Rotavirus gastroenteritis managed in hospitalIn Spain, the annual incidence of hospitalization due to RV in children ranges between 120 and 480 cases per 100000 children under 5 years, and the proportion of cases of AGE attributable to RV ranges between 11% and 55% (Table 2). These differences are mainly explained by differences in methodology. Most studies are retrospective with collection of data from discharge summaries, the Conjunto Mínimo Básico de Datos (CMBD, “minimum basic data set”) and in some instances additional information from the Sistema de Información Microbiológica (SIM, Microbiological Information System). Furthermore, the quality of the documentation and the coding schemes used in the CMBD of inpatients in different hospitals could also be a source of discrepancies in these results.9,11,16 Other factors possibly at play are the patient selection criteria, differences in the protocols for hospital admission and the performance of virologic tests with different diagnostic yields.14
Burden of disease caused by rotavirus requiring hospitalization in Spain.
| Study | Geographical area(Period under study) | Population under study | Methodology | Results |
|---|---|---|---|---|
| López-de-Andrés, 200810 | Spain(2001–2005) | Children 5 years and under.N=95054 children admitted due to AGE | Retrospective, based on nationwide CMBD database.Selection of discharge summaries of children under 5 years admitted for AGE of all causes and RVGE.Costs estimated based on DRG. | 17.1% admissions for AGE coded as RVGE (31% if considering only AGE of infectious aetiology).Mean incidence: 135 admissions/100000 children; 182/100000 in last season (2005); 384/100000 in children under 1 year.Costs attributable to RV: 3 million euro (in 2001), 7 million euro (in 2005) |
| Luquero, 200811 | Valladolid (Hospital Clínico)(2000–2004) | Children under 5 years.N=847 children admitted due to AGE | Retrospective study using 2 sources: hospital CMBD and SIM. | % admissions due to RV: 31.6%.Rate of hospitalization: 480 cases/100000 children.Annual cost of hospitalization due to RV: 123262 euro |
| Giménez-Sánchez, 201012 | Spain(January–March 2006) | Children under 2 years.N=1192 that received care for AGE | Prospective study in 25 hospitals and 5 primary care centres. Active search of RV cases in children under 2 years receiving inpatient care for AGE. Detection by immunochromatography (Vikia). | % admissions due to RV: 54.6% in inpatients.Patients with RVGE had more severe illness and required hospitalization more frequently |
| Gil de Miguel, 20069 | Community of Madrid(1999–2000) | Children under 5 years.N=3862 children admitted due to AGE | Retrospective study based on the CMBD (selection de hospitalizations due to AGE) and the SIM (extrapolation of 1of cases attributable to RV). | 17.5% of cases attributable to RV (peak≈40% in January and February).Annual incidence of hospitalization due to RV: 120 cases/100000 children (220 cases/100000 in children under 1 year).Annual cost of hospitalizations attributable to RV: 565907 € |
| Téllez, 200813 | Castellon (Hospital General)(1995–2004) | Children under 14 years.N=19743 samples of children with AGE | Retrospective study with 10-year followup of test results of the microbiology laboratory of the hospital. Detection of RV by latex agglutination test (Biomerieux®). | % of hospitalizations due to RV: 11.4% |
| Cilla, 2010a14 | San Sebastian, Tolosa and Urola-Costa(1996–2008) | Children under 5 years.N=1290 Children under 5 years admitted due to AGE | Retrospective study. Selection of cases of admission due to AGE; identification of RV+ cases through the electronic database of the microbiology laboratory.Detection of RV by means of EIA (IDEIA™). | Proportion of inpatients with AGE that tested RV+: 39.2% (44.9% in children under 2 years) (3.4% of those requiring intensive care).Annual incidence of hospitalization: 453/215 per 100000 children under 2 years/under 5 years, respectively |
| García-Basteiro, 201115 | Catalonia(2003–2008) | Children under 5 years.N=10.655 children admitted due to AGE | Retrospective study based on the CMBD database. Selection of hospitalizations of children under 5 years with a diagnostic code for RVGE. Costs estimated based on the DRG. | Annual incidence of hospitalization: 104 cases/100000 children under 5 years.22% of hospitalizations due to AGE coded as RVGE.Hospitalization costs associated with RVGE: €431593 (2003) and €809224 (2008) |
| Nosocomial infection | ||||
| Gil-Prieto, 200916 | Spain(1998–2007) | Children under 5 years.N=10990 hospitalised children with secondary diagnosis of de RV | Retrospective study based on the CMBD database.Selection of cases with diagnosis of RV infection admitted to hospital for other reasons. | Annual incidence: 59 cases/100000 Children under 5 years;0.45 cases/100 hospitalizations in children under 5 years.Estimated cost per hospitalization: 3202€ |
| García-Basteiro, 201115 | Catalonia(2003–2008) | Children under 5 yearsN=892 hospitalised children with secondary diagnosis of RV | Retrospective study based on the CMBD database.Selection of hospitalised children under 5 years with a secondary diagnosis of RV and a primary diagnosis unrelated to AGE. | Annual incidence: 0.25 cases per 100 hospitalised children under 5 years |
| Gutiérrez-Gimeno, 201017 | Valencia(October 2006–March 2007) | Children aged 1–23 months.N=1576 admissions in the paediatrics ward | Prospective study with active search of RV in 3 hospitals.Case definition of nosocomial RV infection: onset of AGE between 48h after admission and 72h after discharge.Detection of RV by ELISA (IDEIA rotavirus)+PCR in case of negative results. | Cumulative incidence in 6 months: 2.8 cases per 100 inpatients (0.48 cases per 100 patient days)Additional cost of prolonged hospitalization: €812.2 |
CMBD, Conjunto Mínimo Básico de Datos, nationwide database of hospital discharge summaries; DRG, diagnosis-related group; EIA, enzyme immunoassay; PCR, polymerase chain reaction; RVGE, rotavirus gastroenteritis; RV+, proportion of cases of AGE coded as rotavirus gastroenteritis; SIM, Microbiology Information System.
In Europe, the rate of hospitalization and the proportion of AGE cases due to RV out of all cases of AGE are higher compared to the figures reported for Spain (300–600 cases/100000 children under 5 years and up to 69% of hospital admissions due to AGE) (https://ecdc.europa.eu/en/publications-data/expert-opinion-rotavirus-vaccination-infancy). Differences between health care systems and surveillance systems preclude the direct comparison of the data, but their presence and the fact that the impact of vaccination against RV in Spain has been similar on AGE of all causes and on RVGE suggests that the identification of hospital admissions attributable to RV by means of the CMBD underestimates the burden of hospitalization.1
Nosocomial rotavirus infectionTwo studies have assessed the incidence of nosocomial infection by RV obtaining data from the CMBD database (Table 2), with the limitations inherent in the use of this source to analyze nosocomial infection. The incidence ranged from 0.25 to 0.45 cases per 100 hospitalised children under 5 years. On the other hand, another study with a prospective design found an incidence that was 6–11 times higher.17 In the latter, the authors performed an active search for cases extending to up to 72h after discharge, which allowed a more specific identification of cases and a more accurate estimation of the frequency of nosocomial infection.
A systematic review conducted in Europe found an estimated median incidence of 6.2 cases per 100 hospitalizations (range, 0.3–27.7).18 The European Centre for Disease Prevention and Control (ECDC) has estimated that 25% to 30% of infections by RV in hospitalised children are acquired in hospital, and that these infections are the leading cause of nosocomial diarrhoea in the paediatric population (https://ecdc.europa.eu/en/publications-data/expert-opinion-rotavirus-vaccination-infancy). This adds to the burden of the disease, as it prolongs hospitalizations due to other causes.
Clinical characteristics of rotavirus gastroenteritisIn agreement with what has been described in other countries, the clinical presentation of RVGE in Spain is more severe and associated with a higher incidence of complications compared to diarrhoeas of other causes (Table 3), thus involving a greater use of health care resources, higher costs and a greater psychosocial impact.6
Clinical characteristics of infection by rotavirus in Spain.
| Study | Geographical area(Period under study) | Population under study | Methodology | Results |
|---|---|---|---|---|
| García-Magán, 201419 | Santiago de Compostela (Hospital Clínico)(November–March 2009–2010 and 2010–2011) | Children under 5 years that presented in the emergency department with AGE or developed AGE during hospitalization.N=51 | Prospective cohort study. Collection of clinical data by survey of parents and of microbiological data by stool culture. | RV detected in 53% pf cases.Coinfection in 14% of cases.Increased proportion of hospitalization (63% vs 37.5%), mean length of stay (6.7 vs 4.5 days) and incidence of dehydration (62.9% vs 45.8%) in cases of RVGE compared to AGE of other aetiologies. |
| Redondo-González, 201620 | Castilla-La Mancha(2003–2009) | N=17.415 cases of hospitalization due to AGE | Retrospective based on the CMBD database.Selection of cases with discharge diagnosis of AGE. Identification of diagnostic codes corresponding to associated complications (dehydration, acidosis, hypoglycaemia, seizures and fluid therapy) and comorbidities.Assessment of association by means of logistic regression models. | 10% of cases of AGE coded as RVGE. Coinfection in 18% of cases of RVGE.Association between hospitalization due to RV and place of residence, age, season (P<.0001), dehydration (OR, 12.44; 95% CI, 1.52–40.38), intravenous fluid therapy (OR, 1.74; 95% CI, 1.29–2.35), metabolic acidosis (OR, 1.51; 95% CI, 1.24–1.83), respiratory tract infection (OR, 1.6; 95% CI, 1.09–1.98) and concomitant AGE (OR, 1.52; 95% CI, 1.03–2.25).Dehydration 4 times more frequent in patients under 5 years with RV (OR, 4.36; 95% CI, 1.20–12.96). |
| Arístegui, 20166 | Catalonia, Andalucía and Basque Country.(December 2013–April 2014) | Children 3 years and under seeking care for AGE.N=1087 cases of AGE and 376 cases of RVGE | Prospective study in 31 primary care paediatric clinics and 2 hospital emergency departments. Identification of RV in stool samples by immunochromatography (VIKIA Rota-Adeno). Collection of data for demographic, clinical and environmental variables in a data collection form (researchers) and a patient diary (parents). | Higher proportion of children with RVGE presenting with dehydration at the time of diagnosis (20.3% vs 7.6%; P<.001) as well as greater severity of illness.During the episode of AGE, and compared to children with AGE of other aetiologies, children with RV infection were more likely to have fever (60.1% vs 49.8%; P=.03) and vomiting (65.0% vs 48.0%; P=.001), made more visits to the emergency department (41.5% vs 25.0; P<.001) and required hospitalization more frequently (6.3% vs 33.3%; P=.007). |
CMBD, Conjunto Mínimo Básico de Datos (minimum basic data set), nationwide database of hospital discharge summaries.
In 2006, on the occasion of the introduction of the rotavirus vaccines, the EuroRotaNet surveillance network was established in Europe to track the circulation of the different RV genotypes in participating countries (http://www.eurorota.net). Spain has participated in this network, contributing approximately 700 samples each year.
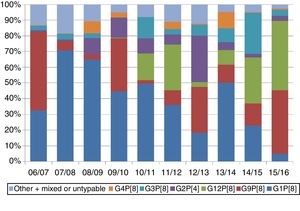
Overall, genotypes G1P[8], G2P[4], G3P[8], G4P[8], G9P[8] and G12P[8] have circulated with a frequency of 1% or greater. In Spain, these 6 genotypes account for 98% of samples in which only 1 genotype is detected. There are seasonal fluctuations in the genotype distribution (Fig. 1). This variability has been described in similar terms in Spanish studies.13,21–24
Based on the reports of EuroRotaNet, there is no evidence to date that rotavirus vaccination programmes are causing an emergence of new genotypes that are not covered by the vaccines. Changes in genotype distribution must be interpreted in the context of the natural fluctuations that are also observed in countries without vaccination programmes and that also existed prior to the introduction of the vaccines.
Economic and family impactTwo studies have evaluated the economic impact of RVGE on families (Table 4). Their results are not comparable, as they were conducted in different periods in relation to the introduction of vaccination.
Economic and family impact of infection by rotavirus in Spain.
| Study | Geographical area(Period under study) | Population under study | Methodology | Variables/outcomes |
|---|---|---|---|---|
| Martinón-Torres, 200825 | Galicia(Hospital Clínico Santiago de Compostela)(December 2005–April 2006) | Children under 2 years.N=88 with AGE (60.5% RV+) | Prospective.Assessment of nonmedical costs incurred before, during and after diagnosis by means of interview with parents and telephone calls. | Indirect costs of RVGE 2.6 higher compared to other aetiologies (€427 vs €163). Increase in transport and food expenses. |
| Bouzón-Alejandro, 2011a26 | Asturias and Galicia(October 2008–June 2009) | Children under 5 years.N=682 con AGE (30.4% RV+) | Prospective study in primary care centres, emergency departments and hospitals (Regalip network).Assessment of nonmedical costs by interviews with parents and an online questionnaire. | Costs of RVGE 1.74 greater compared to other types of AGE (€192.7 vs €111.6), mainly associated with parents missing work. |
| Giménez-Sánchez, 200828 | Spain(February–March 2006) | Children under 2 years.N=1087 cases of AGE (1006 inpatients and 81 managed in primary care) | Cross-sectional study in 25 hospitals and 5 primary care centres.Assessment of severity by Clark severity score.Data collection by means of questionnaire completed by parents. | Greater percentage of severe AGE cases in RV+ patients (30% vs 11.9%).Infection by RV associated with greater anxiety in parents, higher frequency of night awakenings, increased tiredness, more rushing and more stress. |
| Díez-Domingo, 201229 | 3 European countries, including Spain(November 2005–May 2007) | Children under 5 years.N=213 RV+ managed in primary care centres | Prospective study in 15 primary care centres.Severity assessed by means of Vesikari score.Assessment of quality of life by means of a validated questionnaire. | RVGE associated with greater worry, anxiety and interference with everyday activity of parents.Greater severity associated with increased worry and stress. |
| Arístegui, 20166 | Catalonia, Basque Country, Andalucía(October 2013–April 2014) | Children aged≤3 years.N=1087 cases of AGE, with 376 cases of RVGE | Prospective study in 64 primary care paediatrics clinics and 2 hospital emergency departments.Collection of data for variables related to family impact through a patient diary (filled by parents). | RV+ children are significantly more tired, likely to cry and irritable compared to children with AGE of other aetiologies. Parents are more worried and experience more disturbances in their work.Family costs significantly higher in cases of RVGE (€47.3 vs €36.7; P=.011).The frequency of household contacts that were infected was also higher in cases of RVGE. |
RV+, cases of AGE with positive result of rotavirus detection test.
A systematic review of the economic and psychosocial impact of infection by RV in Europe that includes Spanish studies estimated the cost of RVGE episodes treated on an inpatient basis, in the emergency department or in primary care at 1000–1700 euro, 409 euro and 166 euro, respectively, resulting in a total cost for the health care system of 28 million euro a year. The review found that 68% of parents of children hospitalised due to RV missed work, for a mean of approximately 4 days. The estimated annual cost of RVGE to society would rise to approximately 50 million euro.27
Infection by RV also has a considerable psychosocial impact, causing stress on the parents, whose quality of life is affected27 (Table 4). When it comes to prevalent diseases, variables related to quality of life are considered very relevant outcome measures, especially in relation to the interference of paediatric diseases in the everyday activity of households.28 This emotional and social impact may be an intangible cost and pose challenges to its standardization and quantification, but it should nevertheless be considered in the evaluation of health related quality of life in the patients and caregivers affected by the disease.28
There is evidence of an economic and emotional impact in the parents of children with RVGE, either hospitalised38 or managed at the primary care level, and this impact is greater compared to the impact found in parents of children with AGE of other aetiologies.6,29
Vaccination against rotavirusEffectivenessThe reviewed studies found that RV vaccines were very effective in preventing episodes of RVGE and related hospitalizations, and the observed effectiveness was consistent with the efficacy observed in clinical trials32 (Table 5).
Studies conducted in Spain that assessed the effectiveness of vaccination against rotavirus.
| Study | Geographical area (period under study) | Methodology | Effectiveness of vaccination against rotavirus | |
|---|---|---|---|---|
| Decrease in episodes of RVGE (%)(95% CI) | Decrease in hospitalizations due to RVGE (%)(95% CI) | |||
| Martinón-Torres, 201130 | Asturias and Galicia.(October 2008 –June 2009) | Prospective study in primary care centres and hospitals. Analysis of cases and controls.Children under 2 years. | 91.5%(83.7–95.6%) | 95.6%(85.6–98.6%) |
| Castilla, 201231 | Navarra (2008–2011) | Case-control study based on electronic health records and vaccination registry data.Children under 5 years. | 78%(68–85%) | 83%(65–93%)96% (85–99%) between January 2010 and June 2011 |
| Bellido-Blasco, 201232 | Castellon (Hospital General)(2009) | Caso-control study based on test results in the microbiology laboratory of the hospital and the regional vaccination registry.Children under 3 years. | 87.7%(45.5–99.7%) | 93.5% (30.7–99.3%) |
| Pérez-Vilar, 201533 | Valencian Community.(January 2007-June 2012) | Retrospective cohort study with information obtained from health care databases (CMBD and vaccination registry). | - | 84% (75–90%) for RV191% (84–95%) for RV5(effectiveness adjusted based on confirmed cases of hospitalization due to RV) |
| Giménez-Sánchez, 201534 | Almeria(Hospital Torrecárdenas) and El Ejido (Hospital del Poniente)(2005–2013) | Prospective study of cases and matched controls. | - | 86% (59–95%) vs control group A (hospitalizations due to RV– AGE)88% (68–95%) vs control group B (hospitalizations for reasons other than gastrointestinal illness) |
The effectiveness in preventing hospitalizations due to RV ranged between 83% and 96%, with disparities probably stemming from differences in methodology, the post-vaccination periods under analysis, the protocols applied to hospital admission in cases of AGE and the different tests used for determining the aetiology, which differ significantly in their sensitivity.
Studies in Europe have found a similar effectiveness of vaccination, with reductions in the cases of RVGE managed in primary care or at the inpatient level of 68% to 98% depending on the control group used for comparison.35
Economic and clinical impact of vaccinationWe assessed the impact of vaccination against RV by estimating the reduction in the rate of hospitalization using data obtained mainly from hospital databases or electronic health records (Table 6). When comparing data from different sources, it is important to take into account differences in study design, geographical area and vaccination coverage (including how coverage has been estimated), as well as potential differences between hospitals in clinical practices.
Clinical and economic impact of vaccination against rotavirus in Spain.
| Study | Geographical area (period under study) | Study population | Methodology | Variables/outcomes |
|---|---|---|---|---|
| Martinón-Torres, 201236 | Galicia.(July 2003-junio 2010) | Children under 5 years.N=3564 cases of AGE and 1989 of RVGE | Retrospective study using the CMBD database.Comparison of the incidence of hospitalization due to RVGE before (2003–2007) and after (2008–2010) the introduction of vaccination.Estimated vaccination coverage (based on number of distributed doses): 43% in July 2007-June 2008, 51% in July 2008-June 2009 and 46% in July 2009-June 2010. | Reduction in rate of hospitalization due to RV of 44.5% in the July 2009-June 2010 period compared to the pre-vaccination period (July 2003-July 2007) and of 57.3% in infants aged less than 1 year.Reduction of 49.0% in the frequency of hospitalization due to AGE of all causes in the July 2009-June 2010 period, and of 58.7% in infants aged less than 1 year. |
| Gil-Pietro, 201337 | Spain.(January 2005-December 2009) | Children under 5 years.N=111738 admissions due to AGE and 26.500 admissions due to RVGE | Retrospective study using the CMBD database. Comparison of the incidence of hospitalization due to RVGE before (2005–2006) and after (2008–2009) the introduction of vaccination.Estimated vaccination coverage (based on number of distributed doses): 17% in 2017, 35% in 2008 and 38% in 2009. | Reductions in the rate of hospitalization due to AGE of all causes, diarrhoea of unknown aetiology and RVGE pf 35%, 37% and 36%, respectively.In infants aged less than 1 year, the corresponding reductions were of 42%, 43% and 42%. |
| Redondo, 201538 | Castilla La-Mancha.(January 2003-December 2009) | Children under 5 years.N=6593 cases of hospitalization due to AGE and 1640 cases of RVGE | Retrospective study using the CMBD database. Assessment of incidence and its seasonal patterns before (2003–2005) and after (2007–2009) introduction of vaccination.Estimated vaccination coverage (based on the number of distributed doses): 18% in 2007, 39% in 2008 and 44% in 2009). | Decrease of 28% in las the rate of hospitalization due to RV in the 3 years following the introduction of vaccination, with a reduction of 37% in children under 2 years.Reduction of 22% in hospitalizations due to AGE of unspecified aetiology. |
| Giménez Sánchez, 201634 | Almeria (Hospital Torrecárdenas and El Ejido (Hospital del Poniente).(January 2006-December 2013) | Children under 2 years.N=1298 hospitalizations due to AGE | Retrospective study with review of health records of hospitals in geographic areas with a different vaccination coverage (77.1% in Almería and 44.8% in el Ejido in 2009; based on the number of distributed doses).Comparison of rates of hospitalization in the periods before (2005–2006) and after (2007–2013) the introduction of vaccination. | Decrease in the rate of hospitalization due to RV of 23% and due to AGE of all causes of 30.4%. Decrease in relation to vaccination coverage: greater in Almería (H. Torrecárdenas) than in El Ejido (Hospital del Poniente) (RR, 1.67 vs 1.23). |
| Orrico-Sánchez, 20171 | Valencian Community.(January 2002–September 2015) | Children under 5 years born January 1–December 31, 2002. | Retrospective study with use of different databases (SIP; CMBD; Regional Vaccination Registry-SIV).Analysis of the risk of hospitalization due to AGE and RVGE in relation to the vaccination coverage (estimated based on data from the Vaccine Information System [SIV]) and impact on hospitalizations due to AGE in the periods before (2003–2006) vs after (2008–2014) the introduction of vaccination. | Decrease in the rate of hospitalization due to RV of 67%, 71% and 68% in children aged 0, 1 and 4 years, respectively, with vaccination coverage rates of 40–42%.Decrease in the rate of hospitalization due to AGE of all causes of 32% and 39% in children aged 0 and 3 years. Reduction of costs associated with hospitalization of6 million euro/100000 children in a 7-year period. |
CMBD, Conjunto Mínimo Básico de Datos, nationwide database of hospital discharge summaries.
Thus, in their study, Orrico et al. estimated vaccine coverage based on the regional vaccination register of the Valencian Community, unlike other studies in which it has been estimated based on the number of doses of vaccine distributed in the corresponding province, which could be a source of bias. Orrico et al. also fitted a model to control for factors that could modify outcomes (for instance, changes in admission protocols) and calculated the decrease in the rate of hospitalization taking into account the vaccination coverage, establishing the correlation between these two variables and finding that with vaccination coverages of less 20%, the decrease in the risk of hospitalization due to RV was of 37% and 45% in children aged 1 and 2 years, whereas coverage rates of 40% or greater achieved decreases in hospitalization of nearly 70 in the same age group.1 This suggests that intermediate vaccination rates achieve a herd immunity effect. The authors estimated a reduction in the costs associated to hospitalization due to AGE of 6 million euro per 100000 children under 5 years over a period of 7 years.1
Since infection by RV is not a notifiable disease in Spain and its diagnosis does not lead to changes in the treatment of AGE, hospitals may not routinely perform diagnostic tests for its detection. Furthermore, the introduction of the vaccines may have led to an increased use of tests for detection of RV, which in turn could have increased the proportion of cases of RVGE that are actually detected, which would have a negative impact when the effectiveness of vaccination in reducing hospitalizations is assessed based on case counts. Thus, it is important to assess the impact of vaccination not only in terms of hospital admissions due to RVGE, but also taking into account admissions due to AGE of all causes. A study by Redondo et al.38 found that in an estimated 66% of hospital admissions due to AGE, the diagnosis was coded as unspecified AGE. Evidence of age and seasonal distributions of aetiology suggests that a significant proportion of these cases could be cases of RVGE. The studies summarized in Table 6 found a significant reduction not only in cases of RVGE but also in the frequency of hospitalization due to AGE of all causes.
The results of a model to assess the economic impact of universal vaccination against RV with the pentavalent vaccine suggested based on data from a birth cohort suggest that the costs associated with RVGE would decrease by 76%, similar to the decrease observed in other European countries.8 This reduction would amount to annual savings of 22 million euro for the health care system and 38 million euro for society overall. We ought to emphasize that since these are estimates obtained from a model, they must be interpreted with caution.
Despite the significant estimated economic burden of RVGE and the impact of vaccination, cost-effective analyses conducted in Spain39,40 have not found vaccination to be efficient, unlike in other countries.41 Some authors attribute this discrepancy to the cohort study designs used to develop these models in Spain, which lead to underestimation of the indirect impact of vaccination, since they do not take into account herd immunity or undiagnosed cases of RV infection. In Australia, a cost-effectiveness analysis of vaccination that took into account data on herd immunity and the reduction in hospital admissions for AGE of undetermined aetiology observed after the introduction of routine vaccination found a substantially greater reduction in cost compared to the cost estimated in models of disease prior to the introduction of vaccination.42 On the other hand, these studies did not consider changes in the cost of vaccination, which is one of the variables with the highest impact on the analysis of cost-effectiveness.27
Vaccine safetyVaccines against RV have proven to be safe and well tolerated.43 The summaries of product characteristics describe an increased risk of intussusception that was detected in post-authorization surveillance, estimated to amount to 6 additional cases per 100000 children in the 7 days after administration of the first dose. The risk seems to be associated with the age at which the vaccine is administered, so the European Society for Paediatric Infectious Diseases recommends administration of the first dose of vaccine between age 6 and 12–15 weeks, preferably at 6–8 weeks.43
In a study conducted in Valencia that included 136 confirmed cases of hospitalization due to intussusception, 35 (26%) occurred in vaccinated children, and 3 of the latter occurred between 1 and 7 days after administration of the first dose of RV vaccine, corresponding to an adjusted incidence rate ratio of 4.7 (95% CI, 0.3–74.1).44 The authors concluded that the small number of cases of intussusception detected despite the long period under study (5 years) in a country where based on previous studies the baseline incidence of intussusception is high was encouraging. In addition, the incidence of hospital admission due to intussusception has been declining in Spain after the introduction of the vaccines against RV.44
Other lines of researchOther aspects related to RV infection and vaccination against RV that have been studied in Spain include:
- –
The potential impact of vaccination on the microbiota: a comparison of the composition of the microbiota in children aged 12–15 months vaccinated with 3 doses of RV5 and the microbiota of unvaccinated children did not find any differences in the long term.45
- –
The impact of vaccination against RV on hospital admissions due to seizures: the studies conducted in Spain have obtained contradictory results,46,47 so this is an area that requires continued investigation through high-quality studies designed to appropriately assess effect size.
- –
Reliability of diagnosis of RV: one study estimated that there are up to 51% of false-positive results in vaccinated children when immunochromatography is used for diagnosis during periods when the prevalence of disease is low, due to a reduction in the positive predictive value. Interpreting such false-positive results as cases of vaccine failure may lead to a lack of confidence in the vaccine and therefore to a decrease in vaccination coverage.48 It may also have an impact in the estimation of the effectiveness of the vaccine, which would be artificially deflated.
The studies conducted in Spain about AGE-RV and vaccination against RV have yielded precise epidemiological data that corroborate the significant burden of disease and can be used to address the uncertainties about vaccination that prevailed when the vaccines were first authorized in 2006 (Table 7).
Available information (2006 vs 2018) and conclusions.
| Available information | 2006 (Recommendations for Vaccination against Rotavirus. Ministry of Health. September 2006; https://www.msssi.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/Rotavirus.htm) | 2018 |
|---|---|---|
| Burden of disease associated with rotavirus | Information obtained mainly from hospitals or surveillance systems such as the CMBD or SIM | The information on the burden of disease, both community-acquired and nosocomial, has increased considerably through studies in different health care settings, including studies with an active search of cases. Evidence from these studies has revealed an increased severity of AGE in cases caused by RV compared to other aetiologies, and a greater impact on the family and society.We conclude that the burden of disease in Spain is very significant, with a considerable clinical, economic and social impact that was greater than expected before vaccination became available |
| Vaccine effectiveness and impact | Vaccine efficacy data from clinical trials, but no data on real-world effectiveness | Several studies have shown that the effectiveness of vaccination against RV in Spain is very high and similar to the efficacy observed in clinical trials. The effectiveness of vaccination is evinced by the decrease in the number of episodes of RVGE of any severity and the number of hospitalizations due to RVGE.Several studies have found a significant impact of vaccination in the frequency of hospitalization due to RVGE and AGE of all causes, even with an intermediate vaccination coverage |
| Economic impact of vaccination | No data for Spain | The economic impact of vaccination has been estimated based on recent epidemiological data, which shows significant savings both for the health care system and society at large.The cost-effectiveness analyses of vaccination do not take into account the indirect effects of vaccination, undiagnosed cases of RVGE or variations due to changes in the cost of vaccination |
| Protection conferred by vaccines against different genotypes | The data on vaccine efficacy against specific genotypes were limited due to the lower frequency of cases attributable to genotypes other than G1 in the periods that clinical trials were underway | Although studies conducted in Spain have not assessed effectiveness against different genotypes, there is evidence that both the effectiveness and the impact of vaccination have been both significant and sustained through time, independently of fluctuations in circulating genotypes described in genotype distribution studies and reports of the EuroRotaNet surveillance network |
| Pathogenic strain replacement due to vaccine pressure | The diversity of genotypes that can cause disease in humans elicited concern that, as a consequence of vaccination, the most frequently types in circulation would be replaced by others against which the vaccines would not confer protection | Studies on genotype circulation in Spain and especially reports of the EuroRotaNet surveillance network show that despite the diversity of existing genotypes, in the past 10 years 6 genotypes have been most prevalent in Europe and Spain, with no apparent impact of vaccination programmes in the emergence of new genotypes that are not covered by the vaccines |
In the upcoming years, we will find answers to some of the pending concerns related to the extraintestinal manifestations of RV infection and the potential unexpected benefits of vaccination thanks to lines of research that are currently being pursued. In the meantime, the specific body of evidence that has accumulated in Spain in the past decade, in line with the international literature, should prompt the reconsideration of the inclusion of vaccination against rotavirus in the routine immunization schedule, following the recommendation of the leading groups of experts and the example of neighbouring countries.
FundingThe writing of this article was supported in part by a grant from MSD Spain.
Conflicts of interestMGS has collaborated in educational activities sponsored by GSK, Pfizer, Sanofi-Pasteur-MSD and MSD and is currently engaged or has been engaged in the past as a researcher in clinical trials by GSK, Pfizer, Sanofi-Pasteur-MSD, Wyeth and Medimmune.
FGS has received fees to be a speaker in conferences and a consultant for GSK and MSD.
JCR has no conflicts of interest to declare.
FMT has received research grants and/or fees as a consultant/advisor and/or speaker as well as a researcher in the development of clinical trials for vaccines from Abbot, GlaxoSmithKline, Sanofi Pasteur MSD, Merck, Sanofi Pasteur, Pfizer, Novartis, Novavax, Regeneron, Roche, Seqirus and MedImmune Inc.
JDD is the principal investigator in clinical trials by GSK, MSD, Abbot, Pfizer and Sanofi-Pasteu He has also received fees as a speaker and consultant/advisor from Pfizer, MSD, SP and GSK. FISABIO has received research grants from GSK, MSD and SP.
We thank Syntax for Science, Ltd. for its participation in the technical development and processing of this manuscript.
Please cite this article as: Díez-Domingo J, Garcés-Sánchez M, Giménez-Sánchez F, Colomina-Rodríguez J, Martinón-Torres F. ¿Qué hemos aprendido sobre rotavirus en España en los últimos 10 años? An Pediatr (Barc). 2019;91:166–179.