Vein of Galen aneurysmal malformation (VGAM) is an infrequent congenital vascular anomaly (1:25,000 births). It equally affects both sexes and accounts for 33% of cerebral arteriovenous malformations in children.1
The term encompasses a heterogeneous group of vascular malformations whose shared feature is dilatation of the vein of Galen secondary to an arteriovenous shunt. It develops at 6–11 weeks’ gestation as a result of the persistence of the embryonic median prosencephalic vein of Markowski.2–4
Vein of Galen aneurysmal malformations are classified into 2 types: true vein of Galen aneurysmal malformation, which is further subdivided into the mural subtype (fistula in the wall of the vein) and the choroidal subtype (at the subarachnoid level), and vein of Galen aneurysmal dilatation.2,3,5
The clinical presentation is heterogeneous and depends on the age of onset and the size of the malformation. Amacher and Shillito have proposed a classification of clinical presentations based on the age at diagnosis.1 In neonates, VGAM may manifest only with intracranial bruits or congestive heart failure (CHF). In early childhood, onset occurs with macrocephaly, hydrocephalus, CHF and seizures. In older children and adults, it manifests with headache, fainting during exertion and subarachnoid haemorrhage.2,5
The diagnosis can be made prenatally starting from the second trimester of gestation, although the first-line imaging test for diagnosis is magnetic resonance (MR) angiography.4,6
With the aim of learning about the cases that have been managed in our hospital, we conducted a retrospective study of the newborns and infants given a diagnosis of VGAM between January 2004 and January 2017, analysing their epidemiological, clinical, radiological and treatment characteristics.
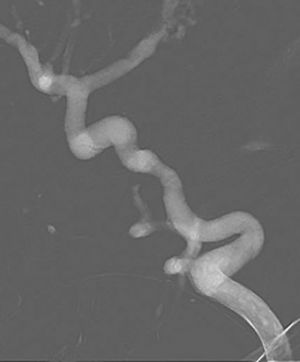
We identified a total of 8 cases, 7 in male patients and 1 in a female patient (Table 1). The diagnosis was made prenatally by ultrasound in 4 patients (34–35 weeks’ gestation) and following the onset of symptoms of CHF in the remaining patients (before age 3 months). The clinical manifestations at birth were macrocephaly in 6; intracranial bruits in 7 and heart murmur in 5. All patients developed CHF and required pharmacological treatment, 4 experienced convulsive seizures (2 after embolization) and 1 developed obstructive hydrocephalus after embolization at age 2 months and required placement of a ventriculoperitoneal shunt. Only one had associated dysmorphic features of unknown aetiology, and the rest had no other malformations. A MR angiogram was performed in all patients. Six patients underwent transarterial embolization (between day 3 and 9 months post birth), and the indication for surgery was unstable CHF in 4 and convulsive seizures in 2. Of these 6 patients, 4 died: 2 due to intraventricular haemorrhage and 2 due to cardiovascular problems. One of the patients died of cardiac arrest before receiving treatment. Only 3 patients survived. One of them did not undergo surgery due to the small size and clinical relevance of the malformation; this patient is currently aged 3 years and remains asymptomatic. The other 2 survivors that underwent surgery are a boy aged 12 years with cerebral palsy and a boy aged 21 months that is asymptomatic at the time of this writing and has undergone 3 embolizations (Fig. 1).
Characteristics of patients under study.
| Sex | Time of diagnosis | Heart murmur | Intracranial bruits | Macrocephaly | Heart failure (age at diagnosis) | Treatment (timing) | Outcome | |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 2 days post birth | No | No | Yes | Yes (3 days post birth) | Embolization (3 months post birth) | Deceased (3 months) |
| 2 | Male | 3 days post birth | Yes | Yes | No | Yes (1 month post birth) | No | Alive (3 years) |
| 3 | Male | 1 day post birth | Yes | Yes | No | Yes (28 days post birth) | Embolization (12 months post birth) | Alive (12 years) |
| 4 | Male | 3 months post birth | Yes | Yes | Yes | Yes (3 months post birth) | 3 embolizations (4–18 months post birth) | Alive (21 months) |
| 5 | Female | Prenatal (34–35 weeks’ gestation) | No | Yes | Yes | Yes (1 day post birth) | Embolization (1 day post birth) | Deceased (1 day post birth) |
| 6 | Male | Prenatal (34–35 weeks’ gestation) | Yes | Yes | Yes | Yes (3 days post birth) | Embolization (1 month post birth) | Deceased (1.5 months post birth) |
| 7 | Male | Prenatal (34–35 weeks’ gestation) | No | Yes | Yes | Yes (2 days post birth) | Embolization (4 days post birth) | Deceased (6 days post birth) |
| 8 | Male | Prenatal (34–35 weeks’ gestation) | Yes | Yes | Yes | Yes (2 days post birth) | No | Deceased (6 days post birth) |
Although this disease is rare, it should be included in the differential diagnosis of any case of heart failure of unknown aetiology in newborns or infants,2 for which auscultation of the fontanel and chest are key elements of the physical examination.
Embolization is a curative treatment, and total occlusion of the malformation is not always necessary to achieve clinical improvement. The timing of surgery depends on the condition of the patient, and the main indication for it is CHF refractory to medical treatment.1 In the neonatal period, the Bicêtre score may be helpful to determine the appropriate timing of surgery.
The prognosis varies based on the size of the aneurysm2 and the age of the patient. In the first months of life, mortality can be as high as 90% without treatment and 52% with treatment.3 Despite the poor prognosis, deferring surgery to at least 6 months post birth is associated with increased survival. However, in many cases clinical instability forces early intervention, which entails an increased risk of morbidity and mortality.
In our case series, we found a high mortality in patients that underwent embolization (66.6%), which could be attributed to the early timing of intervention due to clinical instability, in addition to the difficulties involved in performing endovascular surgeries in newborns. The 2 operated survivors had undergone surgery after age 6 months, and thus had a more suitable anatomy for intervention. We ought to highlight that prenatal diagnosis was not associated with better outcomes, as malformations in these cases were larger, which allowed a better visualization in utero, although it was associated with a slower progression of cardiac manifestations.
Please cite this article as: Ibáñez Beltrán L, García Sánchez JM, Aliaga Vera J, Izquierdo Macián MI. Malformación arteriovenosa de la vena de Galeno. Serie de casos. An Pediatr (Barc). 2019;91:202–204.