Rotavirus (RV) is the leading cause of severe acute gastroenteritis in infants worldwide. Most children are infected by RV by the age of 5years, and especially in the first 2years. Two oral attenuated vaccines against RV are licensed in industrialised countries, which have proven to be safe and effective against the disease.
The main objective of these vaccines has been to reproduce the natural history of infection and protect against severe disease in the first months of life. Preterm infants are at higher risk of severe RV infection compared to full-term infants and infants with normal birth weight.
Data collected on RV vaccination in preterm infants demonstrated that RV vaccines are effective and safe, compared with full-term infants, with a marginal risk of horizontal viral transmission and dissemination when vaccination is performed during hospitalisation.
Preterm infants frequently require admission to hospital after the beginning of the 12th week of life, which suggests that they should receive RV vaccines during admission according to the official immunisation schedule.
El rotavirus (RV) es la causa principal de diarrea infantil grave en todo el mundo e infecta prácticamente a todos los niños en los primeros 5años de vida, sobre todo en los primeros 2años. Existen dos vacunas atenuadas de administración oral frente al RV disponibles en nuestro medio que han demostrado ser seguras y eficaces frente a la enfermedad.
El objetivo principal de estas vacunas ha sido reproducir la historia natural de la infección y proteger frente a la enfermedad grave en los primeros meses de vida. Los recién nacidos prematuros son especialmente vulnerables a la enfermedad por RV, no solo por tener más riesgo de adquirir la infección, sino también por sus complicaciones.
La vacunación frente al RV en niños prematuros ha mostrado resultados de eficacia y seguridad similares a los comunicados en niños a término, y los datos existentes sugieren un riesgo bajo de diseminación e infección nosocomial cuando la vacunación se realiza durante la hospitalización.
Dado que un porcentaje estimable de recién nacidos prematuros permanecen ingresados en las unidades neonatales más allá de las 12semanas de vida, se considera que estos, siempre que su condición clínica lo permita, deben recibir la vacunación frente al RV sin retrasos, incluso durante la hospitalización si así fuese necesario.
Infection by rotavirus (RV) is the leading cause of severe diarrhoea in children worldwide and constitutes a significant public health problem in developed countries. Preterm (PT) infants are a particularly vulnerable population both in terms of the risk of infection by and its severity. At present, 2 vaccines against RV are commercially available in Europe: the pentavalent human-bovine reassortant vaccine (RotaTeq, MSD) and the attenuated monovalent human vaccine (Rotarix, GlaxoSmithKline Biologicals). The existing evidence shows that vaccination of preterm infants against RV is safe and well tolerated and is approximately as effective as it is in infants born at term. Horizontal transmission of the live vaccine virus, while theoretically possible, has not been documented in clinical trials, and the evidence available to date, while having limitations, suggests that the risk of it occurring is low. Preterm infants, especially those born with a weight of less than 1500g, are frequently still hospitalised at the time the first dose is routinely administered, but regardless of inpatient status, if the clinical condition of the infant allows, vaccination should not be delayed.
Impact of infection by rotavirusRotavirus is a ubiquitous pathogen that infects practically all children by age 5 years and is the leading cause worldwide of diarrhoea with dehydration in children aged less than 5 years.1 In Spain, infection by RV causes between 14% and 30% of all cases of acute gastroenteritis (AGE), a fourth of which require hospital admission.2
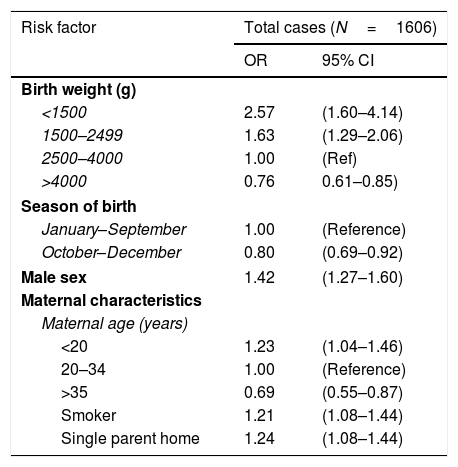
Preterm infants are more vulnerable to RV than their full-term (FT) counterparts, among other reasons due to a diminished transfer of maternal antibodies and a lower breastfeeding rate.3 They are also at increased risk at severe infection by RV, even months after birth, compared to infants born to term and infants with normal birth weights. Severe dehydration, bloody stools and necrotising enterocolitis are more frequent in PT infants compared to FT infants. The increased risk of severe illness due to RV in PT infants was evinced in a study that found that rates of admission due to RV-related diarrhoea in the early months of life were strongly associated with birth weight and increased with decreasing birth weight4 (Table 1). Later on, Dennehy et al.5 analysed the long-term impact of preterm birth in diarrhoea due to RV, and found that a birth weight of less than 2500g was associated with an approximately 3-fold risk of requiring admission in case of AGE caused by RV, even after the early months post-birth, compared to infants with birth weights greater than 2500g (OR, 2.8; 95% CI, 1.6–5.0). The literature also includes descriptions of outbreaks of AGE due to infection by wild-type RV strains in neonatal units.6 It is estimated that nearly ¼ of outbreaks of viral infection in these units are caused by RV,7 and one of the risk factors at play is preterm birth.6
Multivariate analysis of risk factors for hospital admission due to rotavirus-related acute gastroenteritis.
| Risk factor | Total cases (N=1606) | |
|---|---|---|
| OR | 95% CI | |
| Birth weight (g) | ||
| <1500 | 2.57 | (1.60–4.14) |
| 1500–2499 | 1.63 | (1.29–2.06) |
| 2500–4000 | 1.00 | (Ref) |
| >4000 | 0.76 | 0.61–0.85) |
| Season of birth | ||
| January–September | 1.00 | (Reference) |
| October–December | 0.80 | (0.69–0.92) |
| Male sex | 1.42 | (1.27–1.60) |
| Maternal characteristics | ||
| Maternal age (years) | ||
| <20 | 1.23 | (1.04–1.46) |
| 20–34 | 1.00 | (Reference) |
| >35 | 0.69 | (0.55–0.87) |
| Smoker | 1.21 | (1.08–1.44) |
| Single parent home | 1.24 | (1.08–1.44) |
Adapted from Newman et al.4
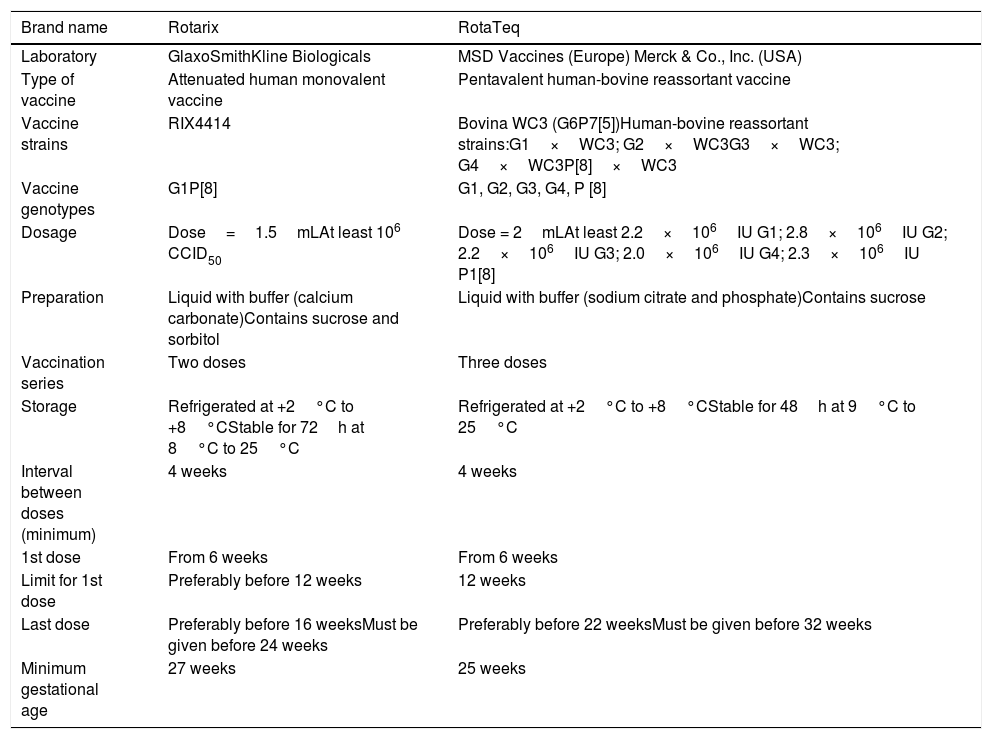
Two vaccines against RV are available in Europe: the pentavalent human-bovine reassortant vaccine (RotaTeq, MSD) and the attenuated monovalent human vaccine (Rotarix, GlaxoSmithKline Biologicals) (Table 2). A meta-analysis of randomised clinical trials has the efficacy of these 2 vaccines is similar in terms of the prevention of severe diarrhoea.8
Available vaccines against rotavirus and vaccination schedule.
| Brand name | Rotarix | RotaTeq |
|---|---|---|
| Laboratory | GlaxoSmithKline Biologicals | MSD Vaccines (Europe) Merck & Co., Inc. (USA) |
| Type of vaccine | Attenuated human monovalent vaccine | Pentavalent human-bovine reassortant vaccine |
| Vaccine strains | RIX4414 | Bovina WC3 (G6P7[5])Human-bovine reassortant strains:G1×WC3; G2×WC3G3×WC3; G4×WC3P[8]×WC3 |
| Vaccine genotypes | G1P[8] | G1, G2, G3, G4, P [8] |
| Dosage | Dose=1.5mLAt least 106 CCID50 | Dose = 2mLAt least 2.2×106IU G1; 2.8×106IU G2; 2.2×106IU G3; 2.0×106IU G4; 2.3×106IU P1[8] |
| Preparation | Liquid with buffer (calcium carbonate)Contains sucrose and sorbitol | Liquid with buffer (sodium citrate and phosphate)Contains sucrose |
| Vaccination series | Two doses | Three doses |
| Storage | Refrigerated at +2°C to +8°CStable for 72h at 8°C to 25°C | Refrigerated at +2°C to +8°CStable for 48h at 9°C to 25°C |
| Interval between doses (minimum) | 4 weeks | 4 weeks |
| 1st dose | From 6 weeks | From 6 weeks |
| Limit for 1st dose | Preferably before 12 weeks | 12 weeks |
| Last dose | Preferably before 16 weeksMust be given before 24 weeks | Preferably before 22 weeksMust be given before 32 weeks |
| Minimum gestational age | 27 weeks | 25 weeks |
When it comes to PT infants specifically, there are data from 2 clinical trials. One of them assessed immunogenicity in 1009 PT infants that received 2 doses of the monovalent RV vaccine or placebo following the scheduled applied routinely to FT infants.9 The rate of antirotavirus IgA seroconversion after the second dose was of 82% in the vaccinated group overall (76% in infants born at 27–30 weeks’ gestation and 88% in infants born at 31–36 weeks’ gestation) compared to 16% in the placebo group. The tolerability of the vaccine was adequate, with a similar frequency of adverse events observed in the vaccinated group and the placebo group (5% vs 7%, respectively).
A post hoc analysis of a placebo-controlled randomised rotavirus efficacy and safety trial compared the use of the pentavalent vaccine against RV and placebo, with administration of 3 doses to a total of 2070 PT infants.10 The efficacy of the vaccine compared to placebo in reducing hospital admissions was of 100% (95% CI, 59–100), in addition to a 92% reduction in RV-attributable emergency department visits (95% CI, 57–100). A nested substudy of 308 premature infants evaluable for detailed safety, the frequencies of adverse events were comparable between vaccine and placebo recipients.
None of the 2 clinical trials in PT infants or of the observational studies that assessed the use of the 2 vaccines detected cases of intussusception.
Observational studies in preterm infantsA prospective study conducted in the United States with administration of the monovalent vaccine to 15 PT infants with intestinal failure found an adequate immunogenicity, with evidence of antirotavirus IgA seroconversion in 12 of the 14 evaluable patients (86%).11 A prospective population-based study conducted in France (IVANHOE study) on the occasion of the new implementation of a routine infant vaccination programme against RV that analysed data for 217 PT infants in the cohort vaccinated with the pentavalent vaccine reported that the number of hospitalizations decreased by a factor of 2.6 in the first 2 epidemic seasons after the introduction of vaccination, and by a factor of 11 in the third season.12,13 Overall, the observational studies published to date have found an adequate safety and tolerability profile for vaccination against RV in PT infants, and these promising results have also been reported in the case series of PT infants with related gastrointestinal diseases, such as necrotising enterocolitis.14,15
A retrospective analysis of the data of more than 1.6 million vaccinations performed between 2001 and 2015 in Michigan (USA) in infants born with normal, low and very low weight found a reduction in the rate of hospitalisation due to RV infection of 91%, 98% and 93%, respectively, and a reduction in the rate of hospitalisation due to AGE compared to unvaccinated children of 62%, 72% and 71%, respectively.16
Dissemination and transmission of the vaccine virus in hospitalised preterm infantsOne of the reasons why the vaccine is used less frequently in hospitalised PT infants is the potential risk of horizontal transmission through the shedding of vaccine virus in faeces and the possible dissemination to other infants admitted to the neonatal unit.
Several studies have evaluated the risk of transmission of RV by vaccinated PT infants to other inpatients. In a study conducted in 2 neonatal intensive care units in Canada that included 102 infants vaccinated with the pentavalent vaccine, the authors analysed the frequency of nosocomial RV infections in a period of more than 2 years preceding the introduction of vaccination and in the first 20 months of vaccination.17 In one of the hospitals, there were no nosocomial RV infections in either the pre- or the post-vaccination periods. In the other hospital, the frequency of nosocomial infection by RV was of 4.9 cases per 10 000 patient-days in the pre-vaccination period (95% CI, 2.0–10.1) and of 0.0 cases (95% CI, 0.0–2.6) in the post-vaccination period. In the study with the largest sample published to date, Monk et al. made a retrospective analysis of the data of 96 PT infants vaccinated with the pentavalent vaccine in the neonatal intensive care unit of a hospital in the United States and of 801 neighbouring infants in the unit who were not vaccinated.18 During the period under study, there was no confirmed case of AGE due to a nosocomial RV infection in the unit.
A study conducted specifically in a neonatal unit analysed the outcomes of vaccination with the pentavalent or monovalent vaccine of a total of 19 vaccinated infants and 49 hospitalised infants whose beds were near those of the vaccinated patients. Testing by real-time polymerase chain reaction (PCR) of the stools of the 49 unvaccinated infants did not detect viral genetic material in any.19 Another study analysed the nosocomial transmission of RV in the paediatric ward, neonatal unit and other inpatient departments in a hospital that vaccinated against RV with the pentavalent vaccine. Of the nearly 1200 stool samples collected during the follow-up, only 13 (1.1%) tested positive for RV by means of real-time PCR: 1 of an unvaccinated child that had a wild-type strain of RV and 12 in vaccinated infants, with no cases of infection by vaccine-type strains in unvaccinated children.20
Thus, horizontal transmission, while possible, has not been documented in clinical studies, and the scarce data on the subject currently available suggests a very low risk. Furthermore, some authors consider that shedding of the virus would be more likely to induce asymptomatic herd immunity than disease.21
Comparison of the risks and benefits of vaccination in the neonatal unitBased on the current evidence, vaccination against RV is considered to offer an adequate cost–benefit ratio for its performance in neonatal units, and therefore the recommendation against vaccination of hospitalised infants before discharge from the neonatal unit that is currently in place in some countries should be reevaluated.21–24 If vaccination is delayed past discharge, some infants may miss the age window for vaccination, which results in a decreased vaccination coverage in this population. Thus, a study conducted in a neonatal intensive care unit in the United States found that 63% of very low birth weight infants did not receive the RV vaccine at discharge, mainly because they were too old to be eligible at that point.14 This is not the only reason for the underuse of vaccination against RV; for instance, in the United Kingdom, where vaccination against RV is recommended and, unlike the United States, the recommendation includes hospitalised infants, a significant percentage of neonatal units do not administer this vaccine.25
Recommendations regarding vaccination against rotavirus in preterm infantsThe official recommendations for vaccination against RV in the United Kingdom emphasise the benefit of vaccination of preterm and very preterm infants, noting that the risk of transmission is low if the usual measures for infection control are implemented, and that the vaccine strain is too attenuated to cause a virulent infection. Thus, the United Kingdom guidelines recommend against delaying vaccination in hospitalised infants as long as they are clinically stable.26
The European Society for Paediatric Infectious Diseases (ESPID) recommends vaccination of PT infants according to their chronological age, including infants born before 32 weeks’ gestation and hospitalised infants, always taking the necessary precautions to prevent transmission to high-risk contacts.27
The Advisory Committee on Immunisation Practices (ACIP) of the United States recommends vaccination against RV of PT infants as long as their chronological age is within the established window, they are clinically stable and, contrary to what was the case in the United Kingdom, vaccination is performed at or after discharge.28
The National Advisory Committee on Immunisation (NACI) of Canada stipulates that the RV vaccine can be given to preterm infants with a chronological age ranging from 6 weeks to 8 months as long as they are healthy and not hospitalised.29 However, the most recent Canadian Immunisation Guide adds that hospitalised infants may be vaccinated after consulting with infection control services and the neonatologist.30
When it comes to vaccination of hospitalised infants, the Australian Technical Advisory Group on Immunisation highlights that the risk of viral transmission is low and therefore vaccination should not be delayed as long as the infant is clinically stable, especially if there is a risk that the infant will miss the age window for vaccination. The advisory group added that if a vaccinated infant requires hospitalisation, no special precautions need to be taken other than the precautions routinely taken to prevent transmission of the virus.31
The Advisory Committee on Vaccines of the Asociación Española de Pediatría (CAV-AEP) has proposed a series of general recommendations for vaccination of PT infants.32 Thus, PT infants should be vaccinated based on their chronological age regardless of their gestational age and weight at birth. It is important to initiate vaccination by 6 weeks post birth and, except in very rare situations, vaccination should not be delayed past the recommended age, administering all vaccines as scheduled in the hospital, if necessary, including the rotavirus vaccine. Preterm infants born before 28 weeks’ gestation or with birth weights of less than 1500g are at increased risk of apnoea and bradycardia in the first 48–72h post vaccination, but these are transient phenomena. The safety of vaccination in PT infants is high, and the reactogenicity is similar to that observed in FT infants. Preterm infants that are still hospitalised must be clinically stable and not exhibit any cardiorespiratory abnormalities at the time of vaccination. Vaccination against rotavirus of hospitalised PT infants has proven safe with the implementation of appropriate hygiene measures, and therefore should be a routine practice in neonatal units once infants reach 6–8 weeks of chronological age if they are stable.2 As a precaution, when the vaccine is given to infants born at or before 28 weeks’ gestation, especially those with a history of lung immaturity, the need for close monitoring for 48–72h following vaccination should be considered.
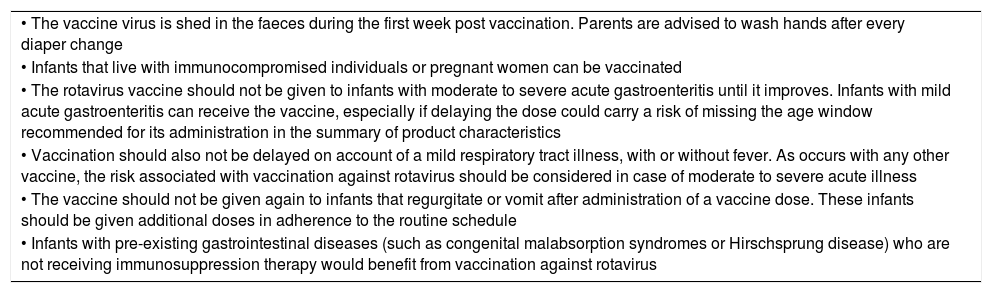
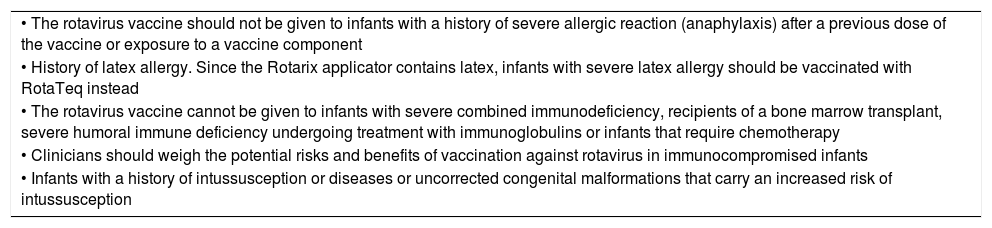
Simultaneous administrationThe rotavirus vaccines can be administered at the same time as other inactivated vaccines included in the primary vaccination schedule. They can also be administered during breastfeeding, feeding of banked human milk or receiving intermittent or continuous enteral nutrition. Tables 3 and 4 present the precautions to be taken and the contraindications for this practice.
Precautions for vaccination against rotavirus.
| • The vaccine virus is shed in the faeces during the first week post vaccination. Parents are advised to wash hands after every diaper change |
| • Infants that live with immunocompromised individuals or pregnant women can be vaccinated |
| • The rotavirus vaccine should not be given to infants with moderate to severe acute gastroenteritis until it improves. Infants with mild acute gastroenteritis can receive the vaccine, especially if delaying the dose could carry a risk of missing the age window recommended for its administration in the summary of product characteristics |
| • Vaccination should also not be delayed on account of a mild respiratory tract illness, with or without fever. As occurs with any other vaccine, the risk associated with vaccination against rotavirus should be considered in case of moderate to severe acute illness |
| • The vaccine should not be given again to infants that regurgitate or vomit after administration of a vaccine dose. These infants should be given additional doses in adherence to the routine schedule |
| • Infants with pre-existing gastrointestinal diseases (such as congenital malabsorption syndromes or Hirschsprung disease) who are not receiving immunosuppression therapy would benefit from vaccination against rotavirus |
Contraindications for vaccination against rotavirus.
| • The rotavirus vaccine should not be given to infants with a history of severe allergic reaction (anaphylaxis) after a previous dose of the vaccine or exposure to a vaccine component |
| • History of latex allergy. Since the Rotarix applicator contains latex, infants with severe latex allergy should be vaccinated with RotaTeq instead |
| • The rotavirus vaccine cannot be given to infants with severe combined immunodeficiency, recipients of a bone marrow transplant, severe humoral immune deficiency undergoing treatment with immunoglobulins or infants that require chemotherapy |
| • Clinicians should weigh the potential risks and benefits of vaccination against rotavirus in immunocompromised infants |
| • Infants with a history of intussusception or diseases or uncorrected congenital malformations that carry an increased risk of intussusception |
Preterm infants should be vaccinated adhering to the same schedule and precautions applied to FT infants if they are clinically stable and meet the age requirement for vaccination against RV.
If PT infants reach the age limit for administration of the first dose (depending on the vaccine used) while still hospitalised, they can be vaccinated in the neonatal unit taking the following precautions to prevent horizontal transmission during the 2 weeks that follow the administration of any of the vaccine doses:
- ∘
Contact isolation measures (wearing gown and gloves).
- ∘
Handwashing before and after handling the patient.
- ∘
Strict hygiene measures during diaper changes.
- ∘
These measures are also applicable to patients who, having been discharged, are re-admitted to the unit for any reason.
If a PT infant is being fed through a nasogastric tube, the vaccine can be administered through this tube followed by administration of a small volume of physiological saline or milk.
If a vaccinated infant is readmitted to the unit in the 2 weeks following vaccination, standard contact precautions should be established and maintained until 2 weeks after the administration of the vaccine.
The rotavirus vaccine can be given at any time before, during or after administration of any blood products, including those containing antibodies.
At the time of vaccination (at the inpatient or outpatient level), the administration of the vaccine must be recorded (depending on the documentation used in the specific setting, in the patient chart, vaccination card or electronic health record database).
In the case of patients that fall outside the protocol (born after 32 weeks’ gestation) whose parents contribute the vaccine purchased privately, clinicians will administer the vaccine and create the corresponding record.
For infants who have had AGE caused by RV before initiating or completing the vaccine series, it is recommended that they initiate or complete vaccination, as scheduled, as primary infection by RV may only confer partial protection against the causative genotype and not other RV genotypes that may be covered by the vaccine.
Preterm infants born at a gestational age that falls outside the established guidelines: the summaries of product characteristics for these vaccines do not include PT infants born before 25 weeks’ gestation (RotaTeq)33 or 27 weeks’ gestation (Rotarix).34 These populations were not included in the clinical trials performed to obtain marketing authorisation for each of these vaccines. There is no evidence suggesting that these vaccines are less safe in these infants. Since very preterm infants are at higher risk of severe AGE caused by RV, we recommend their vaccination after obtaining consent from the parents.
Final recommendation of the Sociedad Española de Neonatología and the Advisory Committee on Vaccines of the Asociación Española de PediatríaInfection by RV continues to be a significant public health problem in developed countries, and PT infants are at higher risk of both infection and its associated complications.
Vaccination of PT infants against RV has proven as efficacious and safe as vaccination of FT infants, and the data currently available suggest that the risk of viral spread and nosocomial infection associated with vaccination during hospitalisation is very low.
The Advisory Committee on Vaccines of the Asociación Española de Pediatría recommends the inclusion of vaccination against RV for all infants in the routine immunisation schedule of Spain.35
In the meantime, given the increased risk of severe illness in PT infants, we recommend that all infants born PT before 32 weeks’ gestation be vaccinated against rotavirus without delay if their clinical condition allows it and there are no contraindications, even if they are still hospitalised.
We recommend public funding of this vaccine under these conditions as vaccination of a risk group, not excluding the possibility of expanding vaccination to other infants born PT after 32 weeks’ gestation where appropriate.
Conflicts of interestJAA has received research grants and/or honoraria as a consultant/advisor and/or speaker for: GlaxoSmithKline and MSD, and conducted vaccine trials for GlaxoSmithKline.
SAS has no conflict of interest with this article.
CDG has no conflict of interest with this article.
AMM has no conflict of interest with this article.
RGS has no conflict of interest with this article.
HBA has received research grants and/or honoraria as a consultant/advisor for: GlaxoSmithKline.
DMP has received research grants and/or honoraria as a consultant/advisor and/or speaker for: GlaxoSmithKline and MSD.
Please cite this article as: Álvarez Aldeán J, Ares Segura S, Díaz González C, Montesdeoca Melián A, García Sánchez R, Boix Alonso H, et al. Recomendaciones para la vacunación frente al ROTAvirus de los recién nacidos PREMaturos (ROTAPREM). An Pediatr (Barc). 2019;91:205.