Vascular cannulation in the neonatal intensive care unit (UCIN) can cause severe ischaemic complications. In some instances, conservative management of these complications is unsuccessful and they may require pharmacological treatment. We describe a series of four clinical cases with favourable outcomes following use of topical nitroglycerin.
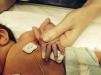
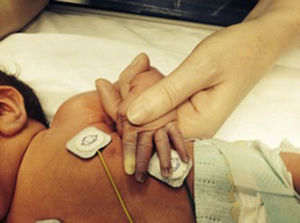
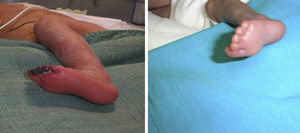
We present the cases of four patients admitted to the NICU, two of them born preterm at 24+6 and 35 weeks, respectively, and two born at term. The extremely preterm newborn underwent cannulation of the umbilical vein and artery, after which he developed pallor in his left leg and eventually necrosis in three toes. The other three patients developed ischaemic lesions in the fingers after placement of percutaneously inserted central catheters and peripheral venous catheters in the upper extremities (Fig. 1). All of them were managed conservatively and their catheters removed. Since there was no improvement, topical treatment with 2% nitroglycerin cream was prescribed (dose of 4mm/kg), which was maintained for 5–18 days, to which all patients responded favourably (Fig. 2). None of the patients experienced adverse effects from the treatment.
Placement of central and peripheral catheters is a common procedure in the NICU. This technique can lead to ischaemic complications, which develope more frequently in patients in critical condition or born preterm. The reason is that their blood vessels are more susceptible to rupturing, vasospasm and thrombosis, which increases the risk of ischaemia and necrosis in adjacent tissues.1
The initial treatment of this complication includes removal of the catheter and application of heat to the contralateral region to promote vasodilation. Occasionally, these measures are insufficient and patients require pharmacological treatment. Research has shown that anticoagulants, thrombolytics or local infiltration of phentolamine or hyaluronidase offer limited benefits and cause side effects when administered systemically.
Nitroglycerin is a nitric oxide donor that has a direct effect as a smooth muscle relaxant, leading to vasodilation of arteries and veins, and consequently improving blood flow after vasospasm or ischaemia.2 Historically, it has been used for the treatment of Raynoud's phenomenon or purpura fulminans. Its use in adults is approved for the treatment of angina, myocardial infarction and arterial hypertension. In the paediatric age group, it has been used off-label to treat chronic anal fissure. In recent years, evidence has been published on the efficacy of nitroglycerin to treat ischaemic complications associated to vascular cannulation or drug extravasation.3
In the cases presented here, nitroglycerin was used as an adjuvant to physical measures. Samiee-Zafarghandy and Mosalli have published several case series and reported similar outcomes with no associated adverse effects, consistent with what we observed in our patients.
Studies in the literature have reported the use of a variety of doses ranging between 0.12mg/kg and 2.5mg/kg. Recent publications have established a dose of 1.22mg/kg (4mm/kg) as safe and efficacious in patients that do not respond to conservative measures. This is the dosage that has been applied in our hospital.3 The topical 2% nitroglycerin formulation was compounded at the hospital pharmacy following current recommendations.
The time to initiate treatment has not been well defined in the literature. In our patients, nitroglycerin was applied in the early hours following the development of ischaemia, but other authors have reported good outcomes with later treatment. The duration of treatment was determined based on the patient's response, and ranged between five and eighteen days, which is consistent with the durations reported in the literature.4
Due to the scarcity of studies and the lack of consensus on the safety and dosage of topical nitroglycerin in children, the use of this drug has been limited for fear of side effects, especially in preterm newborns, who have immature skin and a limited autoregulation of blood flow and therefore are at increased risk of brain haemorrhage. The adverse effects reported most frequently are headache, dizziness, hypotension and methaemoglobinaemia. In our case series, we monitored blood pressure hourly and methaemoglobin daily, and performed a transfontanellar ultrasound before and after treatment with nitroglycerin, and did not observe any significant adverse effects.
Although the available data on the use of nitroglycerin is limited to the description of case series, the outcomes are promising. Few clinical trials have been conducted to assess the use of nitroglycerin in newborns, and we want to emphasise the need to carry out prospective studies with larger samples. Only then will it be possible to standardise prescription regimens for the treatment of arterial vasospasm following vascular cannulation.5
Please cite this article as: Vivar del Hoyo P, Sánchez Ruiz P, Ludeña del Río M, López-Menchero Oliva JC, García Cabezas MA. Uso de nitroglicerina tópica en neonatos con lesiones isquémicas tras canalización de vasos tropical. An Pediatr (Barc). 2016;85:155–156.