Ultrasound (US) guidance increases the success rate and decreases complications during central venous catheterisation (CVC). The benefits of US guidance in arterial catheterisation are less clear. The aim of this study is to compare the outcomes of US-guided arterial catheterisation with the traditional landmark (LM) technique in critically ill children.
MethodsA prospective multicentre study was carried out in 18 Paediatric Intensive Care units in Spain during a 6-months period. Ultrasound guided and landmark techniques were compared in terms of cannulation technical success and immediate mechanical complications.
ResultsA total of 161 procedures were performed on 128 patients (78 procedures in the US group and 83 in the LM groups).. The median (interquartile range) age and weight of the cohort was 11 months (2–52), and 10 kg (4–17) respectively. More than half (59.6%) were male. US was used mainly in high-volume-high-complexity hospitals (cardiac surgery program 76.9% vs. 25.6%, P < .001) as well as in smaller children [weight 5.7 kg (3.8−13) vs 11.5 kg (4.9−22.7), P < .001]. Almost half (49.7%) of the procedures were performed by an inexperienced operator (paediatric resident, or staff with less than 5 years of clinical experience in the PICU), and only 24.4% had performed more than 50 US-guided vascular access procedures before the study. There were no significant differences between US and LM techniques in terms of first-attempt success (35.8% vs 33.7%, P = .773), overall success (75.6% vs 71.1%, P = .514), number of puncture attempts [2 (1−4) vs 2 (1−3), P = .667] and complications (16.6% vs 25.6%, P = .243). Adjustment by potential confounders using multivariate regression models did not modify these results. Subgroup analyses showed that US outperformed LM technique in terms of overall success (83.7% vs 62.7%, P = .036) and complications (10.8% vs 32.5%, P = .020) only when procedures where performed by less-experienced operators.
ConclusionsIn this prospective observational multicentre study, US did not improve arterial cannulation outcomes compared to the traditional LM technique in critically ill children. US-guided arterial cannulation may offer advantages when cannulation is performed by inexperienced operators.
El uso de la ecografía ha demostrado mejorar los resultados de la canalización venosa central. Sin embargo sus beneficios en la canalización arterial en niños no han sido claramente establecidos. El objetivo de este estudio fue evaluar el uso de la ecografía en la canalización arterial en la unidad de cuidados intensivos pediátricos (UCIP).
MetodosEstudio prospectivo multicéntrico en 18 UCIP en España durante un periodo de 6 meses. Se compararon los resultados de la canalización arterial ecoguiada (ECO) con la técnica tradicional basada en referencias anatómicas (REF) en cuanto a la tasa de éxito y las complicaciones inmediatas.
ResultadosSe incluyeron 161 procedimientos en 128 pacientes (78 procedimientos en el grupo ECO y 83 en el grupo REF). La mediana (rango intercuartil) de edad y peso de los pacientes fueron 11 meses (2–52) y 10 kg (4–17) respectivamente y un 59.6% eran varones. La ecografía se utilizó preferentemente en las UCIP de mayor tamaño [número de camas 11 (8−16) vs 6 (4−10), p < 0.001] y complejidad (cirugía cardiaca 76.9% vs 25.6%, p < 0.001) así como en pacientes más pequeños [peso 5.7 kg (3.8−13) vs 11.5 kg (4.9−22.7), p < 0.001]. Un 49.7% de los participantes era personal inexperto (residentes o personal con menor de 5 años de experiencia en UCIP) y sólo el 24.4% de los participantes habían realizado más de 50 procedimientos de canalización ecoguiada antes del estudio. No hubo diferencias significativas entre ECO y REF en la tasa de éxito en una punción (35.8% vs 33.7%, p = 0.773), la tasa de éxito global (75.6% vs 71.1%, p = 0.514), el número de punciones [2 (1−4) vs 2 (1−3), p = 0.667] ni en la incidencia de complicaciones (16.6% vs 25.6%, p = 0.243). El ajuste por variables de confusión en los modelos de regresión no alteró estos resultados. En un análisis de subgrupos se mostró que la ECO mejoró la tasa de éxito global (83.7% vs 62.7%, p = 0.036) y redujo las complicaciones (10.8% vs 32.5%, p = 0.020) en aquellas canalizaciones realizadas por operadores con menos de 5 años de experiencia en UCIP.
ConclusionesEn este estudio prospectivo no hemos observado que, globalmente, el uso de la ecografía mejore los resultados de la canalización arterial en la UCIP. La canalización eco-guiada podría tener ventajas para el personal con menos experiencia.
At present, bedside ultrasonography is an essential technique in the care of critically ill children.1–4 It allows diagnosis, monitoring of the course of disease and more effective and safer performance of invasive procedures at the point of care.4 One of the most studied applications of this technique is vascular access. There is robust evidence that ultrasound-guided vascular access techniques improve the success rate and reduce the incidence of complications in central venous cannulation in both children and adults.5,6 Today, different scientific societies and health care authorities consider that ultrasound guidance should be the standard of care in central venous catheterization.7,8 Ultrasonography has also been used extensively to guide arterial cannulation, and there is evidence supporting its use in radial artery cannulation in adults.9 The data on the use of ultrasound to aid arterial cannulation in the paediatric population is much more scarce. Most paediatric studies have been conducted in stable surgical patients and support the use of ultrasound for arterial cannulation.10 However, the success rates are lower compared to those achieved in venous cannulation.11–13 A probable explanation is that arterial access in children, especially in the youngest ones, requires more experience due to factors such as the smaller caliber of the vessels or the increased risk of vasospasm and thrombosis in this age group.14 All these factors may complicate catheter insertion, even with the use of ultrasound. Few studies have analysed the outcomes of ultrasound-guided arterial cannulation in the paediatric intensive care unit (PICU) setting. In addition to the factors mentioned above, arterial cannulation in the PICU setting is usually performed on an urgent basis in unstable patients, which further complicates the procedure.13
The aim of our study was to compare the success rate and incidence of complications in ultrasound (US)-guided versus anatomic landmark (LM)-guided arterial cannulation.
MethodsStudy design and sampleWe conducted a prospective multicentre study over a period of 6 months (November 2015-April 2016) in 18 PICUs. We included children aged 0–18 years admitted to the PICU that underwent arterial cannulation as indicated by the physician in charge. We only included procedures carried out by the PICU staff. This study was performed in the context of a larger study on vascular access (RECANVA study) organised by the Working Group on Ultrasound of the Sociedad Española de Cuidados Intensivos Pediátricos (Spanish Society of Paediatric Intensive Care, SECIP), whose findings on the subject of central venous cannulation have been previously published .15
Study protocolWe defined US-guided cannulation as the use of real-time US (also known as dynamic US) arterial catheter insertion. We excluded procedures in which US was only used to locate and/or mark the insertion site (static or prelocation approach). We defined LM-guided cannulation as cannulation performed without any form of US assistance in which the insertion site was located by palpating the arterial pulse. We defined an individual procedure as the puncture of an artery using a single technique (US or LM). An attempt was defined as each individual skin puncture performed on the patient. When the first operator was unsuccessful in inserting the catheter, we allowed other operators to attempt cannulation. For the purpose of the analysis, we defined success as placement of the catheter in the artery independently of the number of punctures and the number of operators involved in the process. We defined failed attempt as a procedure in which the operator or operators were unable to insert the catheter, had to switch from one approach to the other (US to LM or vice versa) or the catheter had to be inserted in a different artery than originally intended.
Study variablesFor all patients, we collected data regarding the PICU (number of beds, admissions, care level), the operator (professional qualifications, years of experience), the patient (diagnosis, clinical condition at the time of the procedure, age, weight, sex etc) and the procedure (urgent vs scheduled, artery chosen for cannulation, success rate and complications).
Outcome variablesThe primary outcome was the first-attempt success rate of cannulation. This outcome is habitually used in studies that assess vascular access techniques, as it is considered more sensitive for detection of the advantages of a specific technique over others. Other outcome variables were the overall success rate, the number of attempts, the preparation time (time elapsed from initiation of the procedure to the first puncture), the insertion time (time elapsed from puncture to placement of the guide wire), the reason for failure of the procedure and the incidence of immediate complications (haematoma, accidental venous puncture, ischaemia, thrombosis etc).
Ethical considerationsThe study protocol was reviewed and approved by the competent ethics committee, and we obtained informed consent from the parents of participants.
Statistical analysisWe have expressed quantitative data as median and interquartile range (IQR) and qualitative data as absolute frequencies and percentages. We compared study groups by means of the Mann-Whitney U test and the chi square test. We performed a subgroup analysis, stratifying patients based on the size of the PICU, the experience of the operator, the age of the patient and the vascular access site. We also made a separate analysis of the data of units with a standardised approach for the selection of the cannulation technique, which we defined as performance of 80% or more of the procedures with a single technique. Lastly, we investigated which variables were associated with successful cannulation by means of logistic regression. In addition to group membership (US vs LM), the variables included in the regression model were those corresponding to p-values of less than 0.1 in the univariate analysis. We performed a logarithmic transformation of continuous variables that were not normally distributed. The results are presented as odds ratios (ORs) with the corresponding 95% confidence intervals (CIs). We considered p-values of less than 0.05 statistically significant. The statistical analysis was performed with the software package SPSS version 22 (IBM Corp., Armonk, NY, USA).
ResultsWe included 161 cannulation procedures performed in 128 patients (78 in the US group and 83 in the LM group) with a median age of 11 months (IQR, 2–52) and a median weight of 10 kg.4–17 A total of 18 PICUs participated in the study. Table 1 summarises the clinical characteristics of the patients in the sample. Ultrasound was used more frequently in patients admitted to larger PICUs offering higher levels of care and in patients that were younger and with lower weights. The most frequent diagnoses in both groups were respiratory illness and sepsis/shock. Most US-guided procedures were performed by adjunct physicians employed in the PICU (67.9%), followed in frequency by medical residents (32%), and none by nurses, while in the LM-guided group adjunct physicians performed 48.1% of procedures, followed in frequency by medical residents (30.1%), and a significant number of procedures were performed by nurses (21.6%). Only 24.4% of operators in the US group and 27% in the LA group had performed more than 50 cannulations in the past. In the US group, the most frequent site was the femoral artery (75.6%) with infrequent cannulation of the radial artery (14.1%), while in the LM group there was a higher proportion of radial artery cannulations (39.7%), although the most frequent site was also the femoral artery (46.9%).
Characteristics of the groups.
| US (n = 78) | LM (n = 83) | P | |
|---|---|---|---|
| PICU | |||
| Number of beds | 11 (8−16) | 6 (4−10) | < .001 |
| PICU ≥ 10 beds | 42 (53.8%) | 17 (20.5%) | < .001 |
| Admissions/year | 350 (300−450) | 275 (200−315) | < .001 |
| Level of care | |||
| Cardiac surgery unit | 60 (76.9%) | 20 (24.1%) | < .001 |
| Transplant unit | 57 (73%) | 38 (45.7%) | < .001 |
| Patient | |||
| Sex (male) | 46 (58.9%) | 50 (60.2%) | .796 |
| Age (months) | 5 (1.5−48) | 22 (2.7−81) | .013 |
| Weight (kg) | 5.7 (3.8−13) | 11.5 (4.9−22.7) | .006 |
| Diagnosis | |||
| Respiratory | 39 (50%) | 36 (43.4%) | .400 |
| Sepsis/shock | 33 (42.3%) | 27 (32.5%) | .200 |
| Cardiac | 16 (20.5%) | 7 (8.4%) | .029 |
| Neurological | 4 (5.1%) | 16 (19.2%) | .007 |
| Haematological | 5 (6.4%) | 1 (1.2%) | .081 |
| Renal | 1 (1.3%) | 3 (3.6%) | .342 |
| Trauma | 1 (1.3%) | 10 (12%) | .010 |
| Conditions of procedure | |||
| Urgent procedure | 57 (73%) | 63 (75.9%) | .681 |
| Invasive mechanical ventilation | 69 (88.4%) | 63 (75.9%) | .053 |
| Deep sedation/relaxation | 65 (83.3%) | 67 (80.7%) | .787 |
| Haemostatic changes | 22 (28.2%) | 16 (19.2%) | .181 |
| Previous complications of vascular access | 16 (20.5%) | 10 (12%) | .342 |
| First operator | |||
| Qualifications | < .001 | ||
| Medical resident | 25 (32%) | 25 (30.1%) | |
| Adjunct physician | 53 (67.9%) | 40 (48.1%) | |
| Nurse | 0 (0%) | 18 (21.6%) | |
| Experience in the PICU | .854 | ||
| < 5 years | 37 (47.4%) | 43 (51.8%) | |
| 5−10 years | 24 (30.7%) | 23 (27.7%) | |
| > 10 years | 17 (21.7%) | 17 (20.4%) | |
| Arterial access site | .001 | ||
| Radial | 11 (14.1%) | 33 (39.7%) | |
| Femoral | 59 (75.6%) | 39 (46.9%) | |
| Other | 8 (10.2%) | 11 (13.2%) |
Table 2 presents the outcomes of cannulation. We did not find significant differences between groups in any of the variables under study except for the time to cannulation. While the preparation for the procedure took longer in the US group, the time of insertion was shorter. The most frequent reason for cannulation failure was failure to insert the guide wire despite successful arterial puncture in 44.2% of cases, followed by failure to puncture the artery in 37.2% and complications unrelated to the procedure in 7%. We found significant differences between groups in the reason for cannulation failure. Inability to puncture the artery was the reason for failure in 48% of cases in the LM group compared to 28% in the US group (P < .05), while inability to place the guide wire was more frequent in the latter (US, 55% vs LM, 40%; P = .675). The mechanical complications resulting from insertion or presence of an indwelling catheter were perivascular haematoma perivascular (18%), accidental venous puncture (3.1%), ischaemia of an extremity (0.6%) and arterial thrombosis (0.6%). There were no cases of infection secondary to arterial cannulation.
Outcomes of cannulation.
| US (n = 78) | LM (n = 83) | P | |
|---|---|---|---|
| First-attempt success rate | 28 (35.8%) | 28 (33.7%) | .773 |
| Number of punctures | 2 (1−4) | 2 (1−3) | .667 |
| (≥ 3 puncture) | 29 (37.1%) | 35 (42.1%) | .518 |
| Overall success | 59 (75.6%) | 59 (71.1%) | .514 |
| Cause of failure | |||
| Failure to puncture vessel | 5 (27.7%) | 11 (47.8%) | .027 |
| Failure to place guide wire | 10 (55%) | 9 (39%) | .667 |
| Other | 3 (16.7%) | 3 (13%) | .876 |
| Preparation time (minutes) | 10 (5−15) | 5.5 (5−10) | .002 |
| Insertion time (seconds) | 95 (60−300) | 180 (60−600) | .011 |
| Complications | 13 (16.6%) | 20 (25.6%) | .243 |
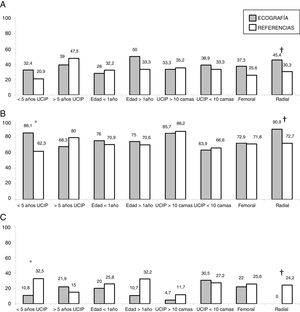
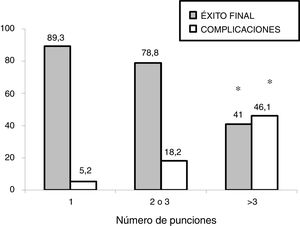
In the subgroup analysis, we found that US guidance only improved outcomes in the procedures performed by PICU staff with less than 5 years’ experience (80 procedures: 37 in the US group and 43 in the LM group), with an increase in the overall success rate (83.7% vs 62.7%, P = .036) and a decrease in complications (10.8% vs 32.5%, P = .020). Outcomes of radial artery cannulation were better in the US group compared to the LM group in terms of the first-attempt success rate (45.45% vs 30.3%; P = .071), overall success rate (90.9% vs 72.7%; P = .096) and the incidence of complications (0% vs 24%; P = .056). Although the differences were not statistically significant due to the small sample size, they are clinically relevant given their magnitude. The subgroup analysis with stratification by PICU size and patient age found no differences between groups (Fig. 1). In the sensitivity analysis in which we only included the procedures conducted in units that used one of the two techniques preferentially (≥ 80% of procedures with the predominant technique) we also found no differences between groups in the outcomes of cannulation. In the multivariate analysis, use of ultrasound was also not associated with any of the outcome variables under study after controlling for possible confounders. The sole variable associated with the first-attempt success rate was the experience of the operator (≥ 5 years vs < 5 years), with an OR of 3.37 (95% CI, 1.13−10.1; P = .029). The only predictor of overall success was the size of the PICU, with an OR of 3.89 (95% CI, 0.88−19.3; P = .006) for a difference in the number of beds of 1 logarithmic unit. The factors associated to the development of complications related to the procedure were the number of admissions per year in the PICU, with an OR of 0.15 (95% CI, 0.04−0.55; P = .004) and the number of punctures, with an OR of 1.52 (95% CI, 1.20−1.93, P < .001) for each additional puncture (Table 3). Fig. 2 shows the association between the number of punctures, the success rate and the complications of arterial cannulation.
Multivariate analysis.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Dependent variable: first attempt success | OR (95% CI) | P | Dependent variable: first attempt success | OR (95% CI) | P |
| Weight | 1.34 (0.94−1.91) | .099 | Operator experience (> 5 years) | 3.37 (1.13−10.1) | .029 |
| Age | 1.19 (0.99−1.42) | .051 | US (vs LM) | 1.55 (0.69−3.48) | .282 |
| Mechanical ventilation | 0.315 (0.136−0.726) | .007 | |||
| Operator experience (> 5 years) | 3.19 (1.51−6.81) | .003 | |||
| [0.1-6]Qualification of operator | |||||
| Resident | Ref | — | |||
| Adjunct physician | 1.74 (0.80−3.78) | .161 | |||
| Nurse | 4.87 (1.57−15.6) | .006 | |||
| US (vs LM) | 1.10 (0.57−2.10) | .773 | |||
| Dependent variable: overall success | OR (95% CI) | P | Dependent variable: overall success | OR (95% CI) | P |
|---|---|---|---|---|---|
| Number of beds in PICU | 3.77 (1.76−8.0) | .001 | Number of beds in PICU | 3.89 (0.88−19.3) | .006 |
| Number of admissions | 3.45 (1.37−8.66) | .008 | US (vs LM) | 1.58 (0.64−3.85) | .313 |
| Cardiac surgery | 3.03 (1.43−6.39) | .004 | |||
| [0.1-6]Qualification of operator | |||||
| Resident | Ref | — | |||
| Adjunct | 0.850 (0.37−1.94) | .715 | |||
| Nurse | 0.282 (0.09−0.883) | .030 | |||
| US (vs LM) | 1.263 (0.626−2.548) | .514 | |||
| Dependent variable: complications | OR (95% CI) | P | Dependent variable: complications | OR (95% CI) | P |
|---|---|---|---|---|---|
| Number of PICU admissions/year | 0.143 (0.046−0.442) | .001 | PICU admissions/year | 0.155 (0.044−0.553) | .004 |
| Age | 0.828 (0.685−1.002) | .052 | Puncture attempts | 1.523 (1.20−1.93) | < .001 |
| Number of puncture attempts | 1.38 (1.154−1.665) | < .001 | US (vs LM) | 1.13 (0.418−3.03) | .810 |
| Experience in PICU (> 5 years) | 0.333 (0.091−1.218) | .097 | |||
| US (vs LM) | 0.63 (0.289−1.379) | .835 |
For the multivariate analysis, the table only shows variables that were statistically significant (P < .05) and comparisons between the US and LM groups.
Arterial cannulation is a frequent procedure in the PICU setting, where it is mainly used for continuous monitoring of blood pressure and collection of blood samples. Although it is a frequently used procedure, it is not free of complications, the most common of which are haematoma, puncture of adjacent structures, ischaemia and thrombosis.16,17
In our prospective multicentre study, the success rates and complications of US-guided cannulation were similar to those of the traditional approached based on palpation of the arterial pulse. This was in opposition to the findings of many previous studies. We ought to highlight that both the first-attempt success rate and the overall success rate were lower compared to studies in which arterial cannulation was an elective procedure performed in the operating theatre.12,18–20 Comparison with previous studies is difficult, as the population of critically ill children differs significantly from the paediatric surgical patient population. Most procedures in the PICU are carried out on an urgent basis and many under unfavourable circumstances: haemodynamic instability, hypotension, haemostatic changes, fluid overload or oedema.
A relevant finding in our study was that the US approach improved outcomes in procedures performed by inexperienced staff. In our study, half of the procedures were performed by staff with little experience in the PICU setting and vascular access. Kantor et al13 carried out a study that included 208 radial artery cannulation procedures performed by staff in training in the PICU. This study, conducted in children with a mean age of 5.8 years, found a greater first-attempt success rate with the use of ultrasound (28% vs 11%; OR, 3.99; P < .001) and a lower failure rate (4% vs 14%; OR, 0.27; P = .032). In addition, the number of punctures required and the time of insertion were also lower in the ultrasound group. Our findings also seem to suggest that ultrasound improves outcomes of radial artery cannulation, although the difference was not statistically significant due to the small sample size. As for operator experience, our study also suggests an improvement with the use of US in the group of operators with less experience. A previous study conducted by our group also found the greatest benefits of US-guided central venous cannulation in adjunct physicians with less experience and medical residents.15 Authors of previous studies have reported similar results.21 It appears that the use of US is particularly indicated in procedures performed by operators with limited experience in vascular access. This rekindles the debate concerning whether training in the classical approach based on anatomical landmarks should be part of the medical curriculum, as some authors have noted that exclusive training in US-guided techniques may result in deficient skills in vascular access when US is not available.22 We also found that, as occurred with central venous cannulation, the number of punctures was the most important factor in the development of complications.20,23 Based on our findings, the number of puncture attempts should be limited to a maximum of 3, regardless of the technique used, in order to preserve the arterial access site.23
In our study, the first-attempt and overall success rates of arterial cannulation were low compared to those of central venous cannulation. In our study, the first-attempt success rate in the overall sample was of 34.7%, similar to the rate reported by Kantor (28.8%) in critically ill children. It appears that arterial cannulation poses challenges in addition to achieving puncture of the vessel. Arteries have a small caliber and a thick muscular wall with a tendency to spasm, which may prevent cannulation even when insertion of the needle tip in the arterial lumen has been achieved. The most frequent reason for failure of cannulation in our study was inability to insert the guide wire. While US can facilitate arterial puncture, it is unlikely to have an effect on the rest of the cannulation procedure, which may partly explain why the evidence in support of US-guided arterial cannulation is so contradictory. Future studies should analyse additional factors, such as the materials used for puncture (angiocatheters, needles, etc) or the type of guide wire.
There are several limitations to our study. Although ours was a multicentre study, the sample of procedures was small. As is the case of any observational study, there was a risk of bias due to the presence of confounding factors. In this instance, we ought to highlight that US was used preferentially in higher-level units and younger children. This may have limited our power to detect benefits of US, as establishing arterial vascular access in these patients is more complicated a priori. In addition, as we noted in the discussion of differences in age between the study groups, we found an uneven distribution of radial artery cannulation, a site used more frequently in older children (median age, 49 months; median weight, 22 kg), and femoral artery cannulation, used more frequently in infants (median age, 4 months; median weight, 5 kg). We attempted to minimise this bias by carrying out different subgroup analyses, stratifying patients based on factors that could affect the outcome of cannulation. While success rates were higher in larger, higher-level PICUs, we did not find any differences based on the cannulation method. Similarly, we also found no difference in the success rate based on patient age. We did find that radial artery cannulation outcomes improved with the use of US, although these differences were not statistically significant. In addition, the analysis of PICUs that used one of the approaches preferentially (in ≥ 80% of procedures) where, therefore, the type of patient or clinical situation had little effect on the selection of the technique, did not detect any differences either. Lastly, it would have been useful to include some form of assessment of the quality of the arterial pulse. In our study, we did not have information on arterial blood pressure, the body mass index or the presence of fluid overload, all of them factors that can affect the quality of the arterial pulse and thus also affect the success rate of cannulation.13
Previous studies show that the use of US in the PICU is increasing in frequency, and it has been proposed that US should be included in the educational curriculum of paediatric intensivists.24–28 However, in Spain there is no accredited training or certification curriculum.28,29 We expect that as training in US improves and its use becomes more widespread, the outcomes of the different applications of this technique, including vascular access, will improve. While evidence from other fields supports the use of ultrasound, it is important to avoid extrapolating results obtained in other settings to the care of critically ill children. Therefore, a necessary step in the future is the performance of high-quality studies, ideally clinical trials, in the PICU setting that would allow establishing the indications and outcomes of the different applications of US in this population.
ConclusionsIn this prospective multicentre study, the overall outcomes of US-guided cannulation were not better compared to the conventional approach of pulse palpation. Ultrasound guidance may be particularly useful when cannulation is performed by inexperienced personnel or in the radial artery. Our findings must be interpreted taking into account the limited experience in the use of US guidance and the heterogeneous characteristics of the participating units and patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Oulego-Erroz I, Mayordomo-Colunga J, González-Cortés R, Sánchez-Porras M, Llorente-de la Fuente A, Fernández-de Miguel S et al. Canalización arterial ecoguiada o por palpación del pulso en la unidad de cuidados intensivos. An Pediatr (Barc). 2021;94:144–152.