Regulatory agencies are responsible for defining the use of off-label (OL) and unlicensed (UL) drug prescription in neonatal intensive care. However, these regulatory criteria may differ between agencies in different countries. The aim of this study was to establish the frequency of OL and UL drug prescription in a sample of patients in a neonatal intensive care unit applying the criteria of the Food and Drug Administration (FDA) of the United States and the Agência Nacional de Vigilância Sanitária (ANVISA) of Brazil, analysing the differences observed in the results based on the applied criteria.
MethodsProspective cohort study in neonates admitted for more than 24h to the NICU of a teaching maternity hospital between August 2017 and July 2018. We obtained information concerning the drugs included in the analysis of OL and UL prescriptions from the DrugDex-Micromedex® and official information on pharmaceutical products in Brazil. We used the kappa correlation coefficient to assess the agreement between the FDA and ANVISA criteria. We defined disagreement as a kappa value of less than 0.200.
ResultsWe evaluated 220 neonates admitted to the NICU and 17,421 items prescribed during the study period. We did not find a difference in the proportion of neonates in which at least 1 drug was prescribed under OL conditions applying the FDA versus the ANVISA criteria (96.4% vs. 98.6%). We found differences between the FDA and ANVISA in the OL classification based on the authorized age of use and indications for prescription, mainly in systemic antimicrobials and cardiovascular drugs. When we compared the prescribing information provided by the FDA and the ANVISA, we found that the criteria of the ANVISA were less specific.
ConclusionOff-label and unlicensed drug prescription are frequent in neonatal intensive care applying the criteria of either agency, although the FDA has established more detailed criteria in terms of the ages and indications for which prescription is authorized.
Las agencias reguladoras son responsables de enmarcar la prescripción de fármacos en condiciones off-label (OL) y unlicensed (UL) en los cuidados intensivos neonatales. No obstante, los criterios de aprobación establecidos pueden variar entre las agencias de distintos países. El objetivo del estudio fue determinar la frecuencia de la prescripción de fármacos OL y UL en una muestra de pacientes en cuidados intensivos neonatales según los criterios establecidos por la Food and Drug Administration (FDA) de Estados Unidos y la Agência Nacional de Vigilância Sanitária (ANVISA) de Brasil, analizando las diferencias en los resultados obtenidos con los criterios de cada agencia.
MétodosEstudio prospectivo de cohortes en neonatos ingresados más de 24 horas en la unidad de cuidados intensivos neonatales (UCIN) de un hospital universitario materno infantil entre agosto de 2017 y julio de 2018. Se obtuvo información sobre los fármacos incluidos en el análisis de prescripción OL y UL de las bases de datos DrugDex-Micromedex® y de la información oficial sobre fármacos de Brasil. La concordancia entre los criterios de la FDA y de la ANVISA se evaluó por medio del índice kappa. Los valores de kappa < 0,200 se consideraron indicativos de discordancia.
ResultadosSe evaluó a 220 neonatos ingresados en la UCIN y 17 421 prescripciones de fármacos individuales en el período de estudio. No hubo diferencias en la proporción de pacientes que recibieron al menos una prescripción OL según se aplicasen los criterios de la FDA o los de la ANVISA (96,4% vs. 98,6%). En cambio, se encontraron diferencias en el motivo de clasificación como OL en base a la edad o a la indicación entre las dos agencias reguladoras, principalmente en la prescripción de antiinfecciosos sistémicos y agentes cardiovasculares. Al comparar las condiciones de prescripción aprobadas por la FDA y la ANVISA, se observó que los criterios de la ANVISA eran menos específicos.
ConclusionesLa prescripción de fármacos off-label y unlicensed es frecuente en los cuidados intensivos neonatales aplicando los criterios de cualquiera de las dos agencias, los criterios de la FDA son más específicos en cuanto a los grupos de edad y las indicaciones para los que se autoriza el uso de los fármacos.
The use of drugs in Neonatal Intensive Care Units (NICU) is essential for the treatment of the newborn, especially in cases of prematurity.1–4 The administration of multiple drugs is common in NICU, despite the risk of adverse events in neonates.1,4,5 In addition to polypharmacy, another characteristic of drug use in neonates is the lack of clinical trials and specific pharmaceutical forms for this population.3,5 Thus, there are several gaps regarding the safety and efficacy of drugs, in addition to relatively few therapeutic options.
Therefore, it is usual to use approved drugs for older children and adults in neonates, as well as the adaptation of pharmaceutical forms to meet specific situations.5,6 In this context, off-label drugs (OL) are defined as those prescribed outside the standards authorized by regulatory agencies in relation to indication, dose, frequency or route of administration. In turn, unlicensed drugs (UL) are those without registration, imported, contraindicated for neonatal use or that require adaptation in the pharmaceutical form before use.7,8
Regulatory agencies, such as the Brazilian National Health Surveillance Agency (Anvisa) and the American Food and Drug Administration (FDA), are responsible for regulating the use of OL and UL in neonates.9,10 However, these criteria may differ between agencies in different countries, causing a prevalence of OL and UL prescriptions between 5%–90%.11–15 Excessively strict criteria may cause less access to drugs; on the other hand, the exaggerated use of OL and UL drugs in neonates increases the incidence of adverse reactions and medication errors.1,4,5
Among the international regulatory agencies, the FDA stands out as a world reference due to its detailed process of registering new drugs and monitoring after approval of use.9,10,16 In Brazil, observational and longitudinal studies characterizing the profile of use of OL and UL drugs in NICUs are few.17–20 As far as we know, there are no studies comparing the profile of OL and UL drugs in neonates using the Anvisa and FDA criteria. This comparison would be relevant considering the possibility of discrepancies between the different agencies and the potential impacts for the therapeutic approach in neonatology.
The aim of this study were defining the incidence of OL and UL prescriptions in a sample of neonatal intensive care patients according to each regulation separately (FDA or ANVISA) and analysing the divergences between the results. Secondary end points were to identify to which anatomic therapeutic chemical group most commonly administered OL belong.
MethodsStudy designThis study was approved by the Institutional Review Board under number 580.201/2014 and all legal representatives authorized the participation of their children in the study by signing an informed consent form. During a full year, from August 2017 to July 2018, we conducted a prospective cohort study in the NICU of a teaching maternity hospital, which is a referral center for high risk pregnancy, women health, gynecological surgery, and pediatric cardiac malformations. During the study period, all neonates admitted to the NICU were evaluated for inclusion. Inclusion criteria were newborns who were admitted at the NICU for longer than 24h, who had at least one prescribed drug during the stay, and whose parents signed an informed consent form. Infants with more than 28 days of life, newborns with a second admission during the study period, and newborns who were prescribed only with parenteral nutrition, continuous intravenous hydration, oxygen therapy, blood products or electrolytes were excluded.
Data collectionFrom each newborn, data was collected on gender, gestational age (GA), birth weight, admission diagnosis, length of stay, and information on all drugs administered during NICU stay (generic names, indication for use, dosage, frequency and route of administration).
All prescribed medicines were classified according to the Anatomical Therapeutic Chemical (ATC) classification system and categorized as labeled, off-label (OL) or unlicensed (UL) according to the FDA approval criteria, available in the DrugDex-Micromedex® database.25 The Anvisa classification was based on information provided in the leaflets of the medicines, therefore, the names of the drug manufactures were also collected and the Anvisa website consulted.26 The drugs were considered unlicensed if: (1) their use was contraindicated for use in neonates, (2) they were extemporaneous preparations that were manufactured or modified by the nurses, and (3) they were imported drugs. The OL category included all medicines where the prescription showed a difference between the license information for age, indication, dose, frequency or route of administration.
Statistical analysisBased on the average admission rate at the NICU, we estimated that 220 newborns would be included during the study period, to which about 12,000 medication items would be prescribed. This sample size allowed to estimate the true percentage of prescribed UL drugs with an accuracy within 0.9 percentage point and the percentage of off-label prescriptions with an accuracy within 0.8 percentage points, with a confidence of 95%. With a sample of 12,000 prescription items we expected, based on the results of a prior pilot study, that 6.600 (55%) are unlicensed drugs and 3840 (32%) OL drugs. Therefore, the maximum error of the estimates obtained in the group of unlicensed drugs would be less than 1.2 percentage points and in the group of OL drugs would be less than 1.6 percentage points, with 95% confidence. These accuracy levels were considered adequate for the research objectives.
Descriptive statistics are presented for demographic and clinical data as number and percentage or mean±standard deviation. Considering FDA and Anvisa criteria, exact binomial 95% confidence intervals (CI) are presented for the proportion of off-label and unlicensed drugs, the proportion of newborns exposed to these drugs and the number of drugs prescribed per neonate. The statistical analysis was performed using Stata release 11 (Stata Corporation, College Station, TX, USA). The Chi Square test of Pearson was used to compare qualitative variables between independent groups of FDA and Anvisa classification. The level of significance was set at 0.05.
The kappa correlation coefficient was used to evaluate the agreement between the FDA and Anvisa criteria for Off-label (age, indication, dose, frequency and route of administration) and unlicensed drugs. The values of kappa <0.200 were considered non-concordant. For each medicine, prescriptions with divergences between FDA and Anvisa criteria for off-label and unlicensed were presented in absolute and relative frequency.
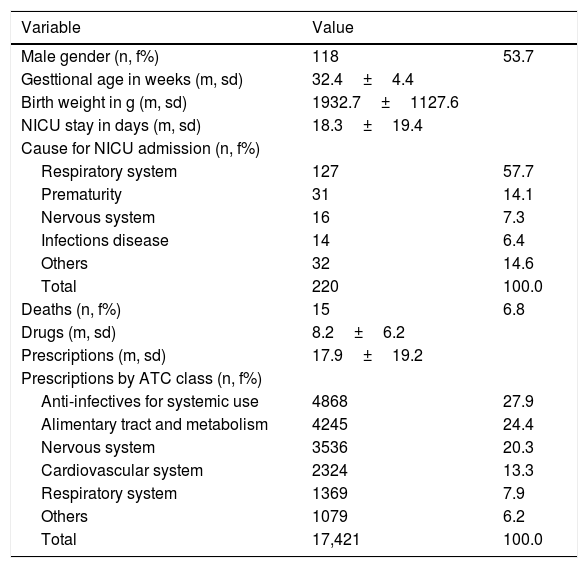
ResultsDuring one year of data collection, we evaluated 3965 prescriptions with 17,421 items prescribed for 220 neonates. There were predominance of male children and preterms with gestational age of 32.4±4.4 weeks (range 23.4–42.4 weeks). The main cause of admission to the NICU were respiratory disorders followed by prematurity, mean hospitalization time was 18.3±19.4 days (median=12 days, range 1–106 days). Antimicrobials for systemic use and medicines acting on the alimentary tract and metabolism were the ATC classes most prescribed, followed by nervous system, cardiovascular system and respiratory system (1369, 7.9%). The Table 1 summarizes the characteristics of the study patients.
Characterization of population and prescribed drugs.
| Variable | Value | |
|---|---|---|
| Male gender (n, f%) | 118 | 53.7 |
| Gesttional age in weeks (m, sd) | 32.4±4.4 | |
| Birth weight in g (m, sd) | 1932.7±1127.6 | |
| NICU stay in days (m, sd) | 18.3±19.4 | |
| Cause for NICU admission (n, f%) | ||
| Respiratory system | 127 | 57.7 |
| Prematurity | 31 | 14.1 |
| Nervous system | 16 | 7.3 |
| Infections disease | 14 | 6.4 |
| Others | 32 | 14.6 |
| Total | 220 | 100.0 |
| Deaths (n, f%) | 15 | 6.8 |
| Drugs (m, sd) | 8.2±6.2 | |
| Prescriptions (m, sd) | 17.9±19.2 | |
| Prescriptions by ATC class (n, f%) | ||
| Anti-infectives for systemic use | 4868 | 27.9 |
| Alimentary tract and metabolism | 4245 | 24.4 |
| Nervous system | 3536 | 20.3 |
| Cardiovascular system | 2324 | 13.3 |
| Respiratory system | 1369 | 7.9 |
| Others | 1079 | 6.2 |
| Total | 17,421 | 100.0 |
n, f%: absolute frequency and relative frequency; m, sd: mean and standard deviation; ATC: Anatomical Therapeutic Chemical Code; FDA: US Food and Drug Administration e Anvisa: Agência Nacional de Vigilância Sanitária, Brasil.
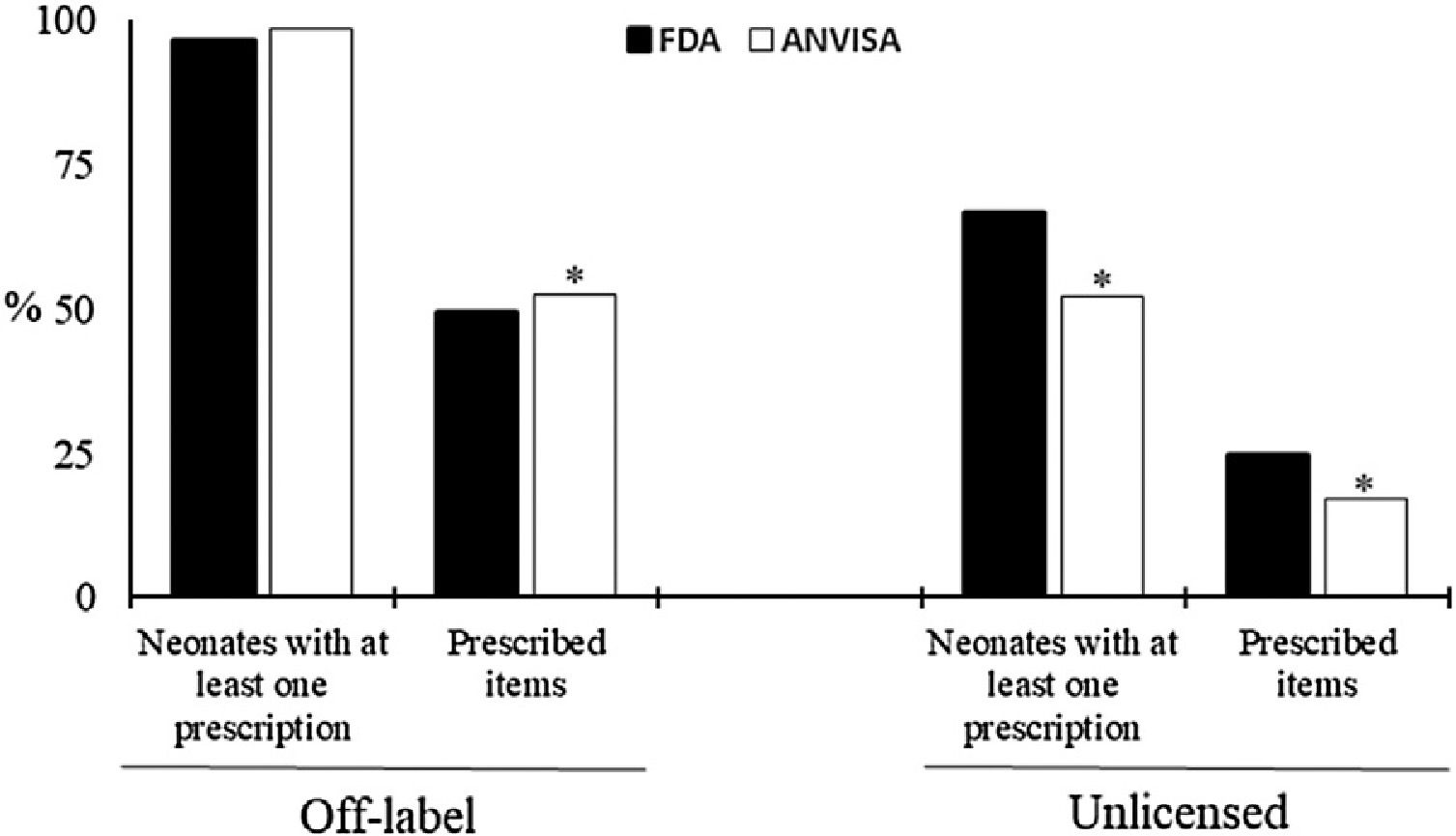
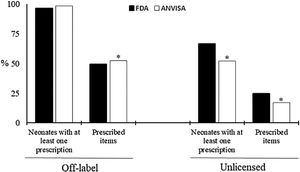
Considering the 220 neonates, the prevalence of children with at least one prescribed OL drug does not differ when using the FDA and Anvisa criteria (96.4% vs 98.6%, P=.13) However, when considering the total of prescribed items (17,421), the FDA criteria indicated a lower occurrence of OL (49.3% vs 52.6%, P<.01) (Fig. 1). In relation to UL, there is a higher occurrence by FDA criteria, both in relation to the proportion of newborns with at least one prescribed drug (66.8% vs 52.3%, P<.01) and the number of prescriptions (24.6% vs 17.2%, P<.01).
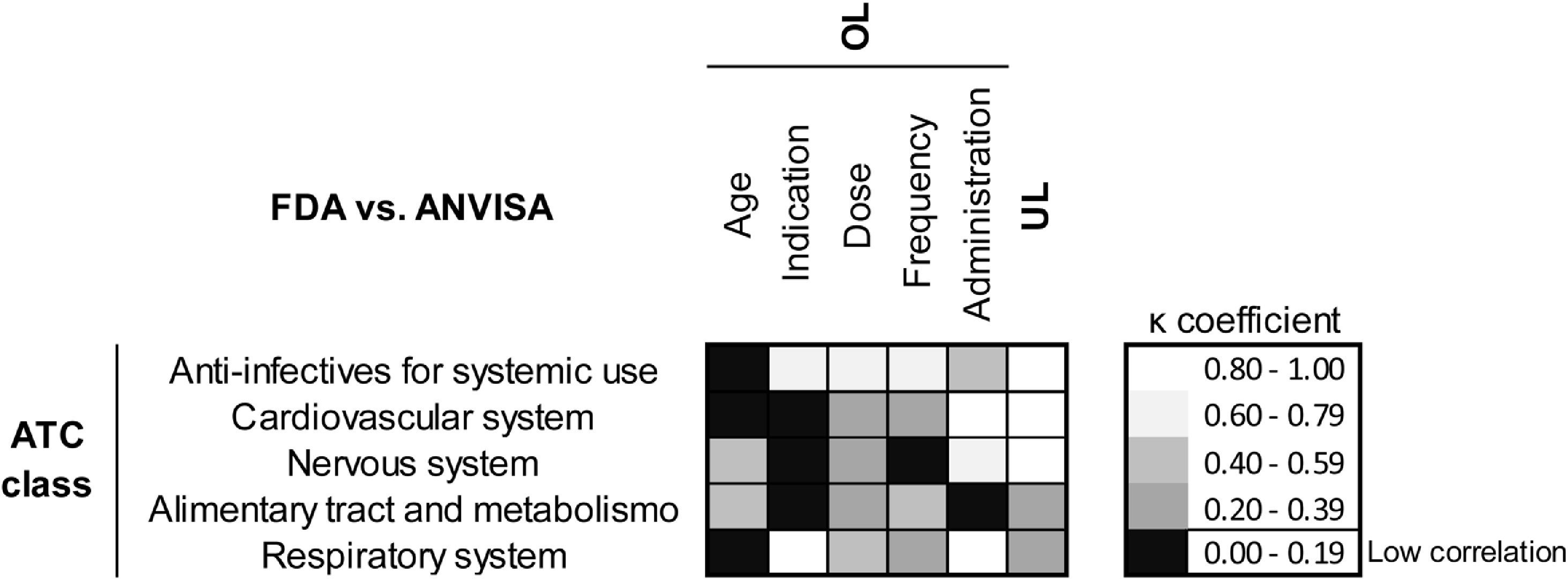
Considering the OL medications, when comparing information from the same drug approved by FDA and Anvisa, we observed significant divergences regarding the age approved for use and indications mainly (Fig. 2). It differs in both regulatory agencies the age indicated for use of antimicrobials for systemic use (k=0.003), cardiovascular drugs (k=0.000) and drugs that act on the respiratory system (k=0.067). However, it is in relation to the clinical indications that the greatest discrepancy is observed in cardiovascular medicines (k=0.004), drugs acting on nervous system (k=0.000) and agents acting on the alimentary tract and metabolism.
Correlation analysis between FDA and ANVISA criteria for classification of Off-label (OL) and unlicensed (UL) drugs in neonatal intensive care. OL medications were evaluated for appropriate age, indication, dose, frequency and route of administration. Values of the kappa correlation coefficient lower than 0.20 (◼) mean low concordance between the American and Brazilian classification systems.
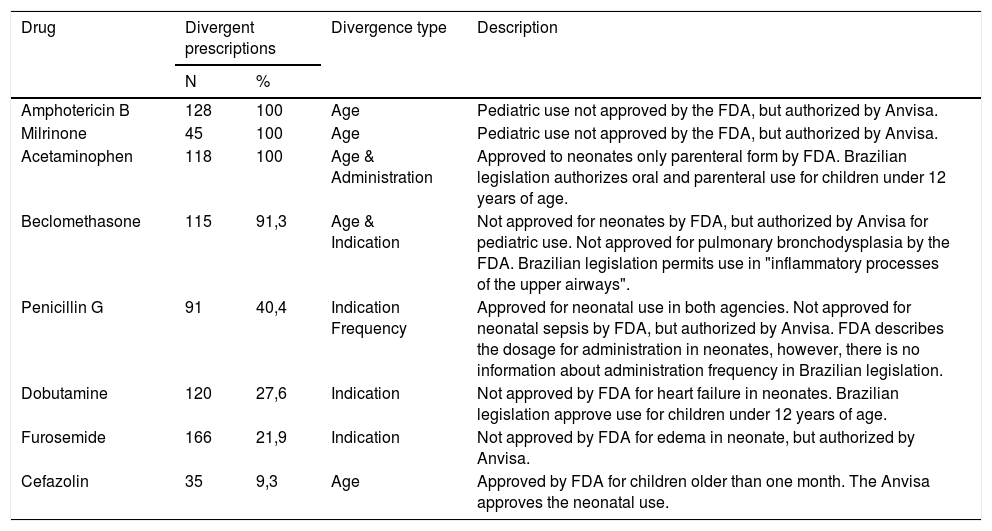
The drugs that most diverge regarding the OL classification criteria (Table 2) include amphotericin B, milrinone, acetaminophen, beclomethasone, penicillin G, dobutamine, furosemide and cefazolin.The evaluation of unlicensed drugs characterized the contraindicated use as the main cause..
Drugs with discordant prescriptions between FDA and Anvisa criteria.
| Drug | Divergent prescriptions | Divergence type | Description | |
|---|---|---|---|---|
| N | % | |||
| Amphotericin B | 128 | 100 | Age | Pediatric use not approved by the FDA, but authorized by Anvisa. |
| Milrinone | 45 | 100 | Age | Pediatric use not approved by the FDA, but authorized by Anvisa. |
| Acetaminophen | 118 | 100 | Age & Administration | Approved to neonates only parenteral form by FDA. Brazilian legislation authorizes oral and parenteral use for children under 12 years of age. |
| Beclomethasone | 115 | 91,3 | Age & Indication | Not approved for neonates by FDA, but authorized by Anvisa for pediatric use. Not approved for pulmonary bronchodysplasia by the FDA. Brazilian legislation permits use in "inflammatory processes of the upper airways". |
| Penicillin G | 91 | 40,4 | Indication Frequency | Approved for neonatal use in both agencies. Not approved for neonatal sepsis by FDA, but authorized by Anvisa. FDA describes the dosage for administration in neonates, however, there is no information about administration frequency in Brazilian legislation. |
| Dobutamine | 120 | 27,6 | Indication | Not approved by FDA for heart failure in neonates. Brazilian legislation approve use for children under 12 years of age. |
| Furosemide | 166 | 21,9 | Indication | Not approved by FDA for edema in neonate, but authorized by Anvisa. |
| Cefazolin | 35 | 9,3 | Age | Approved by FDA for children older than one month. The Anvisa approves the neonatal use. |
No data on medicinal products with a discordant number of prescriptions below 15 for Off-label (epinephrine=10 and gentamicin=12) and unlicensed (ambroxol=7, norepinephrine=1, furosemide=12).
Anvisa provides greater flexibility for pediatric approval when compared to the FDA. Drugs such as amphotericin and milrinone are not released for use in children by the FDA, while beclomethasone and cefazolin may be used in children over one month of age. ANVISA does not restrict the use of these drugs in children under 12 years. According to the FDA, some medications are not released for use under specific conditions such as beclomethasone for pulmonary bronchodysplasia, penicillin G for treatment of neonatal sepsis and furosemide in cases of edema in neonates. However, Anvisa has a wider range of indications for these same drugs (Table 2).
DiscussionThe present prospective cohort study analysed during 12 months the labelling status of drug therapy of neonates hospitalized in a NICU of a Brazilian teaching hospital by both American and Brazilian criteria. The analysis pointed out that almost all neonates in intensive therapy use at least one OL drug, regardless of the regulatory agency consulted. However, considering the total of prescribed items, the occurrence of OL is lower when FDA information is used. Regarding the criteria for classifying OL medications in the two countries, we highlight the discrepancies in relation to age and indications approved for use in neonates. Antimicrobials for systemic use, cardiovascular medicines, drugs acting on nervous system, agents acting on the alimentary tract and metabolism and respiratory system drugs are the most divergent by FDA and Anvisa criteria. In relation to UL drugs, using the FDA criteria, its occurrence is higher when compared to the Anvisa records.
Considering the use of at least one OL and UL drug during the period at NICU, we observed a wide range of prevalence in different agencies. Spanish (Spanish Agency for Medicines and Health Products - AEMPS and European Medicines Agency - EMA), Italian (Italian Drug Compendium) and French (French Medical Regulatory Agency - ASNM) studies point to a prevalence of OL drugs between 22.5%–73.4% and UL 5.0%–53.0%.1,21–27 In the Middle East, countries such as Lebanon, Israel and Palestine also have a wide range of values for OL (35.3%–64.8%) and UL (5.9%–16.0%).28–30 These studies show that the situation varies by country, due to differences in the process of authorization of medicinal products, and in clinical practice. Therefore, although the results are not directly comparable, they do provide a general idea about the subject in question.
However, compared to these studies, our data point to a similarity between Brazilian and American agencies in relation to the prevalence of OL in NICU. When the FDA criteria are used, studies show high prevalence, above 90%.12,30–32 Similarly, in a cohort of 201 neonates, 3290 prescriptions and using the criteria of Anvisa, a prevalence of 95.6% of OL was observed (12). Another Brazilian author shows lower percentages (23.4%), however it is a cross-sectional study with a smaller sample of 73 newborns.15 Therefore, considering only Brazilian cohort studies, there is a similarity between the prevalence of OL by both regulatory agencies, allowing to infer compatibility between Anvisa and FDA.
When considering the total number of prescribed items, our data indicate a higher occurrence of OL according to the Anvisa criteria (52.6% vs 49.3%, P<.05). The FDA is a reference in the world in health legislation, the registration process for new drugs is complemented by rigorous postmarketing monitoring. Specifically in relation to OL use of medicines, American legislation conditions its registration to minimally conclusive scientific results.9 However, clinical trials in neonates are often scarce and over-regulation can make it difficult to use medications in NICU.33 Therefore, the lower occurrence of OL prescription by the FDA criteria in our data can be attributed to the lesser flexibility of the American regulatory agency.
As for UL drugs, there is a decrease in their occurrence when classified by the regulatory agency of the country of origin compared to others. Something expected considering the predominant use of drugs sold in the country and without the need for pharmaceutical adaptation.
In our sample, the main differences between the FDA and Anvisa regarding the OL categorization were age of use and indications. Antimicrobials for systemic use represent the ATC class most commonly used in neonates.34–36 For these drugs, the FDA specifies in detail the age approved for use. For example, according to the FDA, amphotericin B is contraindicated in neonates (Table 2), but authorized by Anvisa for pediatric use (without specifying the age group). Similarly, Anvisa authorizes the pediatric use of medications such as milrinone and acetoaminophen, but without details in cases of neonates.
In the case of penicillin G, both agencies indicate use in neonates. However, the FDA contraindicates in monotherapy for neonatal sepsis, whereas Anvisa generically indicates penicillin G for "infectious processes" in this age group. In general, Anvisa characterizes the indications more broadly when compared to the FDA. For example, FDA contraindicates inhalatory beclomethasone for the treatment of pulmonary bronchodysplasia (PBD) in neonates, but Anvisa indicates its use for "airway inflammatory processes".
The contraindications described involve situations where evidence is scarce and the risk to the neonate is relevant. Amphotericin B is known to be nephrotoxic and hepatotoxic, its toxicity is greater in neonates due to the physiological immaturity that hinders its elimination from the body.37 Penicillin G monotherapy is not indicated due to the high frequency of multi-resistant bacteria in cases of neonatal sepsis. As for PBD, the efficacy of inhaled corticosteroids in neonates over 7 days old is still controversial, in addition to high-potency corticosteroids such as beclomethasone increases the risk of adverse neurodevelopmental outcomes. Although the OL and UL prescription increases the risk of adverse drug events in neonates, its importance is fundamental in the context of NICU. The reduced amount of drugs licensed for use in neonates is due to the scarcity of studies aimed at this population. These represent less than 3% of all studies developed around the planet. In this context, we highlight the discussion of health legislation to enable more options for safe medicines.
This study has some limitations. Firstly, the study was conducted in a single NICU, which may limit generalization of the results. However, the large majority of published studies on this and related topics were also single center studies. Secondly, the data were collected from secondary sources, including clinical charts, nursing records and physician orders, which might decrease data quality, but this was likely to have little impact on the study results because patient data was examined and evaluated prospectively and as was being recorded.
In resume, despite the prevalence of OL medications in NICU being high when evaluated by the FDA and Anvisa, the American agency is more accurate in relation to age and approved indications for use. As for unlicensed use, there is a lower occurrence when classified by Anvisa. Among the regulatory agencies, the most divergent therapeutic classes in terms of age approved for use were antimicrobials for systemic use, cardiovascular drugs and drugs that act on the respiratory system. Regarding the approved clinical indications, the greatest discrepancy is observed in cardiovascular drugs, drugs that act on the nervous system and agents that act on the alimentary tract and metabolism.
We can also conclude that, despite the peculiarities of each country, it is important that the approval of the use of medicines must be subordinated to scientific evidence of quality. However, this implies that regulatory agencies constantly seek current research, in addition to the agility to incorporate this information into clinical protocols. Unfortunately, even considering the FDA as a reference, this process is usually slow and not systematized, which may justify the discrepancies between countries. As perspectives for future research, we suggest investigating whether different classification criteria for OL and UL drugs could result in different clinical outcomes in neonates.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Lima Costa HTM, Peres Florencio A, do Vale Bezerra PK, Chaves Cavalcanti JE, Xavier Costa T, Medeiros Fernandes FE, et al. Evaluación comparativa de la prescripción off-label y unlicensed de fármacos en cuidados intensivos neonatales: guías de la FDA versus guías brasileñas. An Pediatr (Barc). 2021;94:153–160.