Despite treatment with hypothermia, 40% of newborns with hypoxic-ischaemic encephalopathy die or suffer moderate to severe disability. Near-infrared spectroscopy (NIRS) could be a useful, non-invasive tool to establish the prognosis.
ObjectivesTo evaluate the prognostic value of NIRS in predicting neurodevelopmental outcomes at 18–36 months in newborns with hypoxic-ischaemic encephalopathy, and to establish the time points and cut-off values of regional cerebral oxygen saturation that exhibit the strongest correlation to these outcomes.
Patients and methodsThe study included all term newborns with hypoxic-ischaemic encephalopathy managed with hypothermia and NIRS between 2013 and 2016. We established 3 outcome categories: normal neurodevelopment, moderate disability and severe disability.
ResultsThe sample comprised 28 newborns (median gestational age, 39 weeks; median birth weight, 3195 g). The median regional cerebral oxygen saturation increased from 65% to 85% at 48 h post birth. Neurodevelopmental outcomes were normal in 28.6%, while 35.7% developed moderate disability and 35.7% severe disability; 3 patients died. We found a statistically significant difference between groups at 48 h (P = .005) and after hypothermia (P = .03), with higher values in patients with disability. When we compared patients with severe disability group with the other groups, we found a statistically significant area under the ROC curve at 48 h of 0.872 (P = .001) applying a regional cerebral oxygen saturation cutoff of 83.5%. After hypothermia, regional cerebral oxygen saturation values below 66.0% (AUC, 0.794; P = .017) predicted normal development, while values above 82% (AUC, 0.881; P = .001) predicted severe disability.
ConclusionsNIRS seems to be a valuable tool to predict neurodevelopmental outcomes in patients with hypoxic-ischaemic encephalopathy, even after hypothermia, with higher cerebral oxygen saturation values in patients with disability.
A pesar del tratamiento con hipotermia, el 40% de los neonatos con encefalopatía hipóxico-isquémica fallecen o sufren discapacidad moderada-grave. La espectroscopia cercana al infrarrojo (NIRS) se propone como una herramienta útil y no invasiva para establecer el pronóstico.
Objetivosevaluar el valor pronóstico de la NIRS en el resultado del neurodesarrollo entre los 18–36 meses en neonatos con encefalopatía hipóxico-isquémica y establecer el tiempo y los puntos de corte de la saturación cerebral de oxígeno que mejor se relacionan con el pronóstico.
Pacientes y métodosSe incluyeron todos los neonatos a término con encefalopatía hipóxico-isquémica, sometidos a hipotermia y NIRS, entre 2013−2016. Con respeto al resultado, se constituyeron tres grupos: neurodesarrollo normal, discapacidad moderada y grave.
ResultadosSe incluyeron 28 neonatos (edad gestacional mediana: 39 semanas; peso al nacimiento mediano: 3195 gramos). La saturación cerebral de oxígeno tendió a aumentar desde una mediana de 65% hasta 85%, a las 48 horas de vida. El resultado del neurodesarrollo fue normal en 28,6%, el 35,7% adquirió discapacidad moderada y el 35,7% discapacidad grave (tres fallecieron). Se halló una diferencia estadística entre los grupos a las 48 horas (p = 0,005) y después de la hipotermia (p = 0,03), con valores más altos en los pacientes con discapacidad. Teniendo en cuenta el grupo con discapacidad grave frente a los otros, la curva de ROC a las 48 horas resultó en un área debajo de la curva (AUC) significativa de 0,872 (p = 0,001), con un punto de corte de saturación cerebral de oxígeno de 83,5%. Después de la hipotermia, los valores de saturación cerebral de oxígeno por debajo del 66,0% (AUC 0,794; p = 0,017) predijeron el desarrollo normal mientras que valores por encima del 82% (AUC 0,881; p = 0,001) predijeron discapacidad grave.
ConclusionesEl NIRS parece constituir una herramienta útil en la predicción del resultado del neurodesarrollo de pacientes con encefalopatía hipóxico-isquémica, incluso después de la hipotermia, con valores más altos de saturación cerebral de oxígeno en pacientes con discapacidad.
Hypoxic-ischaemic encephalopathy (HIE) associated with perinatal asphyxia is one of the most frequent causes of brain damage in full-term neonates despite advances in medicine.1 In developed countries, the incidence is of 2–6 cases per 1000 live births.2 Early initiation of hypothermia is the only therapy proven beneficial; it reduces brain post-asphyxial reperfusion injury3,4 and it may decrease mortality and the frequency of future disability, including cerebral palsy.5,6
Brain injury may cause behavioural, cognitive, sensorimotor or language impairment and epilepsy.7 Various techniques are available for assessment of brain function in neonates with HIE and to try to predict neurodevelopmental outcomes.1 One of these techniques is amplitude-integrated electroencephalography (aEEG), which seems to be useful for predicting outcomes after birth asphyxia.8,9 It should be started as soon as possible to guide decision-making regarding the initiation of hypothermia, although aEEG results are not necessary for this decision. There is evidence of an association between a normal aEEG pattern and an increased probability of survival without sequelae.2 Therapeutic hypothermia seems to reduce the positive predictive value of aEEG because it delays the normalization of the background pattern and the onset of sleep-wake cycling.1,3,10–12 In addition, aEEG tracings may be affected by the depressant effects of the anticonvulsant and sedative drugs used during hypothermia and by the scalp oedema frequently found in these newborns.10
The findings of magnetic resonance imaging (MRI) have also been shown to correlate to neurodevelopmental outcomes at 12–24 months of age, but only if the scan is performed at least 24 h post birth.13 The MRI scan is important to confirm the diagnosis and to establish a prognosis. However, it cannot be used for continuous monitoring.7
Another possible predictor of neurodevelopmental outcomes that has been investigated less extensively is the regional cerebral oxygen saturation (rScO2) obtained by near-infrared spectroscopy (NIRS). The latter is a non-invasive bedside method used to monitor regional brain oxygenation in neonates in many clinical settings.7 Soon after birth, the rScO2 increases from 40% to 56% to up to 78% in the first two days, and then it stabilizes between 55% and 85%.7 Previous studies have shown that these values are higher in infants with adverse outcomes due to decreased cerebral energy metabolism with decreased oxygen uptake, cerebral hyperperfusion and impaired autoregulation of the cerebral vascular bed with vasodilation following severe brain injury.1,7,8,10,14 There is a dearth of few data on rScO2 values after therapeutic hypothermia.1 Combined monitoring with NIRS and aEEG seems to have a stronger predictive value for long-term neurodevelopmental outcomes.3,9
The aim of this study was to assess the predictive value of NIRS for neurodevelopmental outcome at 18–36 months in newborns with HIE admitted to a paediatric intensive care unit and managed with therapeutic hypothermia.
As secondary objectives, we aimed to establish cut-off rScO2 values for prediction of severe and moderate disability and the timing of rScO2 measurement associated with the best predictive values.
Study sample and methodsWe conducted an exploratory study with retrospective collection of data in the tertiary paediatric intensive care unit (PICU) of the Centro Hospitalar e Universitário de Coimbra, the referral centre for hypothermia in perinatal asphyxia in the Centro region of Portugal. We included all term newborns admitted with a diagnosis of moderate or severe HIE that underwent evaluation with NIRS during and after hypothermia between January 2013 and December 2016. We defined perinatal asphyxia as a 10-minute Apgar score of 5 or less, a duration of ongoing cardiopulmonary resuscitation (CPR) of more than 10 min, a peripheral or umbilical cord blood pH of less than 7.0 and/or a base deficit of at least 16 mmol/L in the first 60 min post birth in a patient with seizures or evidence of moderate or severe encephalopathy on examination by a qualified professional (altered level of consciousness, change in tonus or reflexes or absence of independent breathing).2 The exclusion criteria were birth at or before 36 weeks’ gestation, newborn not managed with hypothermia, presence of major congenital anomalies (such as congenital heart disease), clinical features suggestive of chromosomal abnormalities, inborn errors of metabolism or brain infarction, patient lost to follow-up or missing data in health records.
We collected clinical data from the electronic health records database (SClínico®, B-ICU.Care®, FileMaker®). We analysed the following variables: sex, childbirth complications, gestational age, type of delivery, Apgar score, total duration of cardiopulmonary resuscitation, birth weight, peripheral or umbilical cord blood pH and base deficit in the first 60 min of life, core temperature at admission, time of initiation of hypothermia, aEEG and MRI findings, rScO2 values obtained by NIRS, detection of clinical seizures and length of stay in the PICU.
Hypothermia was delivered with the CritiCool™ system (Belmont Medical Technologies), setting a target core temperature of 33.5 °C to be maintained for 72 h. Rewarming was performed at a rate of 0.2 °C/h. The device used for aEEG monitoring was the Pulmocor® cerebral function monitor, using three channels derived from parietal and frontal leads. We analysed aEEG patterns and epileptic activity. We defined abnormal aEEG pattern as discontinuous normal voltage, a burst-suppression or flat pattern, or a low voltage trace. We documented the worst aEEG pattern found in each patient. The rScO2 was measured with the INVOS™ (Medtronic) using a neonatal sensor. The sensor was placed on the left or right frontal bone of the infant’s head. Although rScO2 values were monitored continuously, we chose to record the values at 12, 24, 36, 48, 72 h and after hypothermia (day 4 or 5 post birth). We recorded the values obtained with the INVOS™ device at these arbitrary time points. We classified detected MRI changes using the MRI score developed by Weeke et al.15
We collected outcome data from the records of clinical evaluations conducted at 18–36 months of life, including data on the following: death, cerebral palsy, epilepsy, sensory abnormalities and findings of the neurodevelopmental assessment.
The neurodevelopmental assessment included the use of 2 instruments. Cognitive development was assessed by means of the Griffiths Mental Development Scales (GMDS). This scale yields a total score and 6 subscale scores (locomotor, personal-social, hearing and language, eye-hand coordination, performance and practical reasoning).16 Adaptive functioning was assessed with the Vineland Adaptive Behavior Scales (VABS). This instrument assesses the cognitive, social, and practical skills acquired by the child to meet the demands of everyday life and has several subdomains (communication, daily living skills, socialization and motor skills).16 We calculated IQ-type standard scores and z-scores for the results in both of these scales. We defined normal development as scores between –1 standard deviation (SD) and +1 SD (standard score, 85–114). We defined global developmental delay as a z-score for 2 or more subscales in one or both instruments below –2SD, which corresponds to a standard score of 70, and borderline developmental delay as a z-score in 2 or more subscales in one or both instruments between –1 SD and –2 SD (standard score, 71–85). These tests were administered by a psychologist with experience in neurodevelopmental assessment.
Cerebral palsy was diagnosed according to the criteria established by the Surveillance of Cerebral Palsy in Europe network (Surveillance of Cerebral Palsy in Europe, 2000).17
Epilepsy was diagnosed applying the 2017 criteria of the International League Against Epilepsy.18
When it came to outcomes, we defined severe disability as death, cerebral palsy with motor functioning at level 3 or higher in the Gross Motor Function Classification System (GMFCS) or higher, global development delay, hearing loss requiring hearing aids, or blindness. We defined moderate disability as borderline developmental delay, mild to moderate sensory abnormalities (hearing or visual impairment without need of hearing aids or blindness) or epilepsy (seizures requiring anticonvulsant therapy at the time of assessment). We defined normal outcome as normal development in the absence of neurological sequelae.
We performed the statistical analysis with the software SPSS® version 24. We calculated measures of central tendency and dispersion for quantitative variables and absolute and relative frequencies for qualitative variables. We compared nominal variables using the chi square test and quantitative variables with the Kruskal–Wallis test (testing the normality assumption by means of the Kolmogorov-Smirnov test). We used the Bonferroni correction on the obtained p-values. We generated receiver operating characteristic (ROC) curves to assess the accuracy of NIRS in predicting neurodevelopmental outcomes and used the Youden index formula to establish the cut-off rScO2 values to optimise the sensitivity and specificity in the prediction of severe disability and normal outcome. We set the level of significance at 5%.
ResultsDuring the study period, 38 newborns were admitted with severe or moderate HIE, of who 28 met the inclusion criteria. We excluded 10 newborns: 1 with congenital heart disease, 2 due to missing data on rScO2 values and 7 because they were lost to follow-up in the first months of life.
Applying the modified Sarnat score, 7 newborns had severe HIE, while the rest had moderate HIE.
The median gestational age at birth was 39 weeks (interquartile range [IQR], 38–40). The median birth weight was 3195 g (IQR, 2975–3515). Of all patients, 71.4% (n = 20) were male. The median Apgar scores were 5 (IQR, 5−7) at 5 min, and 6 (IQR, 6−8) at 10 min. In the first hour of life, the median pH value was 6.9 (IQR, 6.9−7.1) and the median base deficit was –18.9 mmol/L (IQR, 14–23).
There were complications during delivery in 85.7% of cases (n = 24/28): meconium-stained amniotic fluid in 11, umbilical cord entanglement in 6, uterine rupture in 3, placental detachment in 2, fetal-maternal haemorrhage in 1 and maternal acute hypotension and anaemia in 1.
Most newborns were delivered by caesarean section (67.9%; n = 19/28), and 28.6% (n = 8/28) with the use of vacuum extractors or forceps. Only 1 patient was born by normal spontaneous delivery.
Therapeutic hypothermia started a median of 5.5 h post birth (IQR, 3.5−7.4). Passive hypothermia was initiated in all patients before transfer to the hospital. On admission, the core temperature was less than 35.0 °C in 23 of the 24 neonates in which it was documented.
The aEEG pattern was abnormal in 82.1% of the patients (n = 23/28), mainly in the first 3 days of life and after initiation of hypothermia: burst-suppression pattern in 11, low voltage trace in 8 and discontinuous normal voltage in 4. Electrographic seizures were detected in 64.3% (n = 18/28), in absence of clinical seizures in 4 of them. Four patients without electrographic seizures had clinical manifestations compatible with seizures.
The MRI scan, performed at a median postnatal age of 7 days (IQR, 6–11), was abnormal in 67.9% of the neonates (n = 19/28). The median MRI score of the sample was 6.5 (IQR, 1–14) with a maximum of 26.
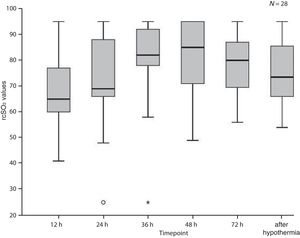
Fig. 1 presents the median rScO2 values of the entire sample during and after hypothermia. The median rScO2 value tended to increase from 65% (IQR, 60–78) at 12 h to a maximum of 85% (IQR, 70.5–95) at 48 h of life, after which they tended to decrease. The values recorded after hypothermia were obtained at a median of 16 h after initiation of rewarming (IQR, 15–21.5) with a median core temperature of 36.4 °C (IQR, 36.2 °C–36.5 °C).
The neurodevelopmental outcome was normal in 28.6% of patients (n = 8/28), while 35.7% (n = 10/28) had moderate disability and 35.7% (n = 10/28) severe disability. Three newborns died (at 6, 9 and 16 days post birth) after treatment was redirected due to severe brain injury and a poor prognosis.
Twenty infants were assessed with the GMDS at a median age of 21 months (IQR, 18–24.7). Twenty-one were assessed with the VABS at a median age of 18 months (IQR, 18–23.5). These scales revealed global developmental delay in 5 patients and borderline developmental delay in 9. Both scales were used to evaluate 64.3% of the infants (n = 18/28). We found cerebral palsy in 5 patients with a GMFCS level 4 or 5. Eight patients had a diagnosis of epilepsy. One patient had moderate hearing loss.
Table 1 presents the clinical and diagnostic workup of the three neurodevelopmental outcome groups.
Clinical and diagnostic workup in each neurodevelopmental outcome group.
| Normal outcome | Moderate disability | Severe disability | P | |
|---|---|---|---|---|
| n | 8 | 10 | 10 | |
| GA, weeks, med (IQR) | 39 (38.2−40) | 39 (37.7−40) | 39 (37−39.2) | .542 |
| BW, grams, med (IQR) | 3160 (2922.5−3495) | 3220 (2957.5−3381.2) | 3180 (2930−3626.2) | .805 |
| Male, n (%) | 4 (50.0) | 7 (70.0) | 9 (90.0) | .174 |
| 5' Apgar score, med (IQR) | 5 (3.2−6) | 5.5 (5−7.2) | 5 (4.5−7) | .352 |
| 10' Apgar score, med (IQR) | 6 (5−7) | 7 (6−8) | 6 (5−8) | .163 |
| pH, med (IQR) | 6.9 (6.8−7.0) | 7.0 (6.9−7.1) | 6.9 (6.9−7.1) | .157 |
| Base deficit, mmol/L, med (IQR) | −18.2 (−15.4 to −23) | −16.6 (−12.1 to −23.3) | −19.4 (−15.2 to −22.6) | .630 |
| Clinical neonatal seizures, n (%) | 1 (12.5) | 8 (80.0) | 9 (90.0) | .001 |
| MRI score, med (IQR) | 0.5 (0−3.5) | 3.5 (1−7.5) | 14.5 (11.7−19.2) | <.001 |
| Abnormal aEEG pattern, n (%) | 6 (75.0) | 7 (70.0) | 10 (100) | .178 |
| Seizures in aEEG, n (%) | 3 (37.5) | 6 (60.0) | 9 (90.0) | .065 |
| Length of stay in PICU, med (IQR) | 8 (7−10.7) | 9 (7−12.2) | 14.5 (10.5−22.5) | .021 |
aEEG, amplitude-integrated electroencephalography; BW, Birth weight; GA, Gestational age; IQR, interquartile range; med, median; MRI, magnetic resonance imaging; PICU paediatric intensive care unit.
We found a statistically significant difference between neurodevelopmental outcome groups in the MRI score, with poorer scores in the severe disability group. Clinical seizures were also significantly more frequent in the moderate and severe disability groups (Table 1).
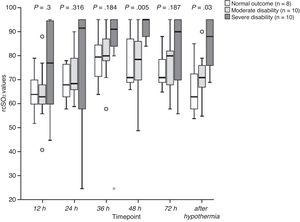
When we compared rScO2 values between neurodevelopmental outcome groups (Fig. 2), we found a statistically significant difference at 48 h of life between the severe and the moderate disability groups (P = .019), and the severe disability and the normal outcome groups (P = .013). There were also significant differences after hypothermia between the severe disability and the normal outcome groups (P = .003) and between the severe and the moderate disability groups (P = .043).
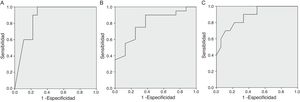
We found a statistically significant area under the curve (AUC) at 48 h for the comparison of the severe disability group with the rest of the patients (AUC = 0.872; 95% confidence interval [CI], 0.742–1; P = .001). A cut-off point of 83.5% corresponded to a sensitivity of 100% a specificity of 72.2%, a positive predictive value (PPV) of 66.7% and a negative predictive value (NPV) of 100%. After hypothermia, the ROC curve showed a statistically significant difference between the normal outcome group and the moderate and severe disability groups combined (AUC = 0.794; 95% CI, 0.614−0.974; P = .017), with an optimal cut-off point of 66.0% (sensitivity, 90%; specificity, 62.5%; PPV, 85.7%; NPV, 71.4%); and between the group with severe disability and the moderate disability and normal outcome groups combined (AUC = 0.881; 95% CI, 0.752–1; P = .001), with an optimal cut-off point of 82.0% (sensitivity, 70.0%; specificity, 88.9%; PPV, 77.8%; NPV, 88.9%) (Fig. 3).
Receiver Operating Characteristic (ROC) curves of NIRS values (A) at 48 h comparing severe disability versus moderate disability or normal outcome; (B) after hypothermia comparing normal outcome versus moderate or severe disability; (C) after hypothermia comparing severe disability versus normal outcome or moderate disability.
In this study, median rScO2 values tended to increase in patients with moderate or severe HIE during hypothermia from 65% to 85% at 48 h of life, with higher values in patients with disability. The differences in rScO2 values between the normal neurodevelopmental outcome, moderate disability and severe disability groups were significant at 48 h and after hypothermia.
Median rScO2 values increased during cooling, probably due to decreased oxygen uptake during hypothermia. The high rScO2 values observed during cooling, even in infants with good outcomes, could also be explained by the use of sedative drugs, which leads to a lower metabolic rate and a decreased oxygen demand.1
In agreement with previous works, our study found higher rScO2 values in patients with poor outcomes, which could be explained by decreased oxygen consumption and lower metabolic rate due to neuronal loss.1,9,14 In a study by Peng et al. in neonates with perinatal asphyxia treated with hypothermia, those who developed brain injury had consistently higher rScO2 values during both the treatment and the rewarming periods. However, the difference between patients with perinatal asphyxia that developed brain injury and those that did not was only significant in the first 10 h of hypothermia treatment.14 In our study, rScO2 values were significantly higher in the severe disability group at 48 h, with values above 83.5% in all, and after therapeutic hypothermia. All patients that had rScO2 values above 82% after hypothermia belonged to the severe disability group. In opposition, almost all patients with rScO2 below 66% after hypothermia had good outcomes.
Toet et al. found that rScO2 values increased past the normal range after 24 h in infants with adverse outcomes. However, these authors only compared rScO2 values until 48 h post birth, and their study was published before the introduction of therapeutic hypothermia.8 In a study in infants treated with hypothermia, Lemmers et al. found that rScO2 values increased in both the favourable and the adverse outcome groups, but this increase was modest in the favourable outcome group compared to the adverse outcome group at 24, 36, 48, and 84 h post birth. These authors used a cut-off value of 77% to define abnormal rScO2 during hypothermia.3 A study by Goeral et al. that only included patients with moderate HIE, the mean rScO2 value did not differ significantly between the groups with normal and pathological MRI findings, although rScO2 values were lower in the normal MRI group, especially after 36 h post birth.9 The AUC for the rScO2 values was greatest between 90 and 96 h.9 Niezen et al. reported that rScO2 values were not associated with outcome during the first 48 h, but that infants with severe outcomes had significantly higher rScO2 values at 72 h post birth and 24 h after rewarming.1 Dix et al. supported the use of cerebral oxygenation monitoring with NIRS during at least the first 3 days of life.7
Our findings corroborate those of previous studies in support of a longer duration of NIRS monitoring, as rScO2 values after hypothermia seem to have a good predictive value. Our cut-off points may help predict neurodevelopmental outcomes. However, based on the current evidence, trends in rScO2 values seem to be more useful than isolated rScO2 measurements.11
In our study, a substantial proportion of infants had poor neurodevelopmental outcomes despite the early initiation of hypothermia in most with a core temperature below 35.0 °C, which is the recommended neuroprotective temperature considered safe until the beginning of controlled therapeutic hypothermia.2 The mortality reported in the literature in patients with HIE managed with hypothermia is higher compared to the mortality in our sample.4,19,20 When it comes to survival with disability, it is difficult to compare results between studies due to differences in methodology, although we ought to mention that a higher percentage of children in the study by the TOBY Study Group survived without neurologic abnormalities compared to our sample. The incidence of cerebral palsy is similar in previous studies,4,19 while the percentage of patients that developed epilepsy is lower compared to our study.19,20,21
As expected, MRI scores were higher in patients that developed moderate or severe disability, which corroborates the good predictive value of this score.15 When it comes to the aEEG pattern, previous studies have not found significant differences, possibly because the monitoring takes place during cooling.1,3,10–12 We ought to mention that in our study, all patients in the severe disability group had abnormal findings, although this was also the case of some patients with a normal outcome, and the proportion of the latter was smaller compared to the MRI score, which further corroborates the good predictive value of this exam.
In our study, electrographic seizures detected by aEEG were more frequent in both disability groups, but this difference was not statistically significant. However, we found a statistically significant difference in the presence of clinical seizures, which were more frequent in the newborns that developed disability. Gucuyener et al. propose that repetitive seizures are associated with a low tissue oxygenation index because the excitotoxic injury leads to the consumption of large amounts of oxygen by neurons,11 and Niezen et al. found that the absence of epileptic activity during cooling was a strong predictor of a favourable outcome.1,9 In this study, we used different tools (aEEG, NIRS, imaging methods) that can assist physicians in making a prognosis when the patient has a complex diagnosis such as HIE. The neurodevelopmental evaluation was made with recognised and validated instruments administered by an experienced psychologist. As recommended in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), the evaluation assessed both cognitive skills and functional behaviour. Developmental delay was the most frequent sequela, but we also found significant proportions of epilepsy and cerebral palsy, which was consistent with the previous literature.22,23 We should also highlight that 9 children had borderline developmental delay before 36 months, which is associated with a high probability of significant learning difficulties and behavioural problems in the future.
There are limitations to our study, chief among which are the small sample size and the frequency of missing data due to its retrospective design. In addition, several variables that we did not take into account, such as haematocrit and blood pressure values, the level of respiratory support and certain medications, are known to interfere with cerebral oxygenation, and therefore constitute another potential source of bias in our study.
Nevertheless, and in conclusion, NIRS seems to be a valuable bedside tool for predicting neurodevelopmental outcomes in patients with HIE treated with hypothermia, and it has the advantage of being noninvasive and allowing continuous monitoring during cooling. This technique is even useful after hypothermia. The use of techniques such as NIRS to predict neurodevelopmental outcomes in neonates with HIE may be important for the purpose of individualising current and future neuroprotective therapeutic strategies. Studies with larger samples and prospective data collection are needed to corroborate these findings.
Previous presentations: This study was presented as an electronic poster at the 30th Annual Meeting of the European Society of Paediatric and Neonatal Intensive Care, June 19, 2019, Salzburg, Austria.
Please cite this article as: Oliveira Pereira C, Diasa A, Nunes Vicente I, Pinto JT, Marques C, Dinis A, et al. Valor pronóstico de la espectroscopia cercana al infrarrojo en la encefalopatía hipóxico-isquémica. An Pediatr (Barc). 2021;94:136–143.