As of May 1, 2020, coronavirus disease caused by infection by SARS-CoV-2 (COVID-19) has affected over 3 181 000 people worldwide and caused more than 220 000 deaths. Around 1% of cases occur in children under 10 years, most frequently with a mild course of disease.1 Cancer patients receiving chemotherapy could be more vulnerable to complications from COVID-19, as suggested by Liang and colleagues,2 but the evidence on paediatric cancer patients is scarce.3
We present the case of a boy aged 20 months that had received a diagnosis of high-risk B-cell acute lymphoblastic leukaemia with KMT2A-MLLT3 rearrangement 2 months prior.
At the end of the second part of the induction phase, the patient developed febrile neutropenia in the absence of respiratory symptoms or any other sign of focal infection. Due to the high prevalence of COVID-19 in Spain, in addition to blood and urine cultures, the workup included a SARS-CoV-2 test by real-time reverse transcription polymerase chain reaction (RT-PCR) in a nasopharyngeal swab sample, which was negative. We initiated empirical antibiotherapy with piperacillin-tazobactam and amikacin in adherence with local guidelines for the management of febrile neutropenia. Twelve hours after admission, the patient developed tachypnoea, and crackles could be heard on auscultation in the left base of the lung. At that point, we performed a second SARS-CoV-2 RT-PCR test, which was positive, while the findings of the chest X-ray were normal. In the hours that followed, the boy developed hypoxaemia requiring supplemental oxygen, and we initiated treatment with oral hydroxychloroquine and azithromycin as suggested by Gautret et al.4Streptococcus mitis was isolated from blood cultures of samples taken at admission, leading to discontinuation of amikacin and initiation of vancomycin. The patient received a transfusion of red blood cells and platelets to manage post-chemotherapy aplasia. He did not exhibit haemodynamic instability, coagulopathy, fluid overload or renal or hepatic failure. For the next 6 days, the boy remained hypoxaemic, with respiratory distress and daily fever, requiring high-flow oxygen therapy (maximum flow of 2 L/kg/min with FiO2 of 30%–40%). A second chest X-ray revealed left basal and hilar condensation.
Given the persistence of febrile neutropenia, we initiated empirical treatment with liposomal amphotericin B, and ruled out invasive fungal infection.
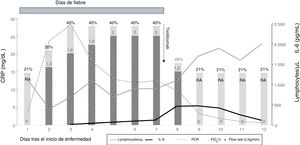
Due to suspicion of cytokine release syndrome (CRS), described elsewhere as a predictor of serious worsening of the patient’s condition,5 we monitored the levels of C-reactive protein (CRP), interleukin-6 (IL-6) and ferritin (Fig. 1), and the patient was given a single dose of tocilizumab, a recombinant humanized anti-human IL-6 monoclonal receptor antibody.
Course from disease onset. The lines represent the lymphocyte count (cells/µL) and laboratory parameters associated with cytokine release syndrome: C-reactive protein (CPR) (mg/dL) and interleukin-6 (IL-6) (pg/mL); the bars represent oxygen therapy (L/kg/min) with the FiO2 (%). After treatment with tocilizumab, the fever resolved, and oxygen therapy was discontinued 24 h later. RA, room air (FiO2 21%).
After the dose of tocilizumab, the fever disappeared immediately and all respiratory symptoms resolved, allowing discontinuation of oxygen therapy 24 h later. The levels of CRP decreased, and haematological recovery started. The levels of IL-6 rose in the first few days, reaching a peak of 478 pg/mL on day 9 of admission (day 2 after administration of tocilizumab) and then decreased on day 3 after tocilizumab administration, as described in some models of rheumatoid arthritis. Ferritin levels continued to increase after administration of tocilizumab, peaking at 1600 ng/mL on day 11 of admission (day 5 after tocilizumab). Other laboratory biomarkers related to CRS such as triglycerides, lactate dehydrogenase or fibrinogen were all normal, as was procalcitonin. We did not detect any side effects related to tocilizumab.
Antibiotherapy ended after completion of a 1-week course. The boy was discharged 14 days after admission following haematological recovery, at which time he was free from COVID-19 symptoms and the findings of the physical examination were normal.
Fourteen days after the symptoms resolved, another RT-PCR test for detection of SARS-CoV-2 in a nasopharyngeal swab sample was performed with negative results, and chemotherapy resumed.
This case illustrates the clinical picture of severe COVID-19 in a paediatric patient with cancer, including the development of CRS following the onset of symptoms directly associated with SARS-CoV-2 infection. Although the concurrent Streptococcus mitis bacteraemia and the platelet transfusions may have played a role in the development of acute respiratory distress syndrome, we suspected CRS because the patient did not show improvement in fever and respiratory symptoms despite appropriate supportive care and antibiotherapy. Furthermore, the complete resolution of fever and respiratory symptoms after the administration of a single dose of tocilizumab fit the pattern described in severe COVID-19 cases in adults.6
To conclude, while most paediatric patients with COVID-19 have mild symptoms, children with cancer may develop severe COVID-19, in which case CRS markers should be evaluated and the use of tocilizumab contemplated after ruling out bacterial and fungal infections. Repeat SARS-COV-2 tests are advisable in cases with high clinical suspicion with initial negative results, especially in immunocompromised patients with severe infection.
We thank Magda Campins and the team of the Department of Preventive Medicine and Epidemiology for their guidance in the management of the disease and their participation in constructive discussions.
Please cite this article as: Velasco Puyó P, Moreno L, Díaz de Heredia C, Rivière JG, Soler Palacín P. Tocilizumab en ni˜no con leucemia linfoblástica aguda y síndrome de liberación de citoquinas asociado a COVID-19. An Pediatr (Barc). 2020;93:132–133.