Despite being an international reference in donation and transplantation, Spain needs to improve pediatric donation, including donation after the circulatory determination of death. The present article, a summary of the consensus report prepared by the Organización Nacional de Trasplantes and the Spanish Pediatrics Association, intends the facilitation of donation procedures in newborns and children and the analysis of associated ethical dilemma. The ethical basis for donation in children, the principles of clinical assessment of possible donors, the criteria for the determination of death in children, intensive care management of donors, basic concepts of donation after the circulatory determination of death and the procedures for donation in newborns with severe nervous system's malformation incompatible with life, as well as in children receiving palliative care are commented. Systematically considering the donation of organs and tissues when a child dies in conditions consistent with donation is an ethical imperative and must become an ethical standard, not only because of the need of organs for transplantation, but also to ensure family centered care.
A pesar de ser una referencia internacional en donación y trasplante, España precisa mejorar los procesos de donación en niños, en particular la donación tras la determinación de la muerte por criterios circulatorios (donación en asistolia). El presente artículo, resumen del documento de consenso elaborado por la Organización Nacional de Trasplantes y la Asociación Española de Pediatría, pretende facilitar los procesos de donación en niños y neonatos y analizar los conflictos éticos que plantea. Se comentan los fundamentos éticos de la donación pediátrica, los principios de la evaluación clínica de los posibles donantes, los criterios diagnósticos de muerte encefálica en niños, los cuidados intensivos para el mantenimiento de los donantes, los conceptos básicos de la donación en asistolia y los procesos de donación en neonatos con malformaciones muy graves del sistema nervioso incompatibles con la vida y en niños en cuidados paliativos. Considerar sistemáticamente la donación de órganos y tejidos cuando un niño fallece en condiciones de ser donante es un imperativo ético y ha de constituir un estándar profesional, tanto por la necesidad de órganos para trasplante, como por asegurar un cuidado integral centrado en la familia.
Spain is an international leader in organ donation and transplantation. In 2019 the organ donation rate was 49 per million inhabitants, with performance of 5449 solid organ transplants, the result of a management system structured to systematically identify potential donors and ensure effective donation.1 There were 163 solid organ transplants from donors under 16 years of age. In the paediatric population, there are several complications at both the donation and the transplantation levels that result in a decreased probability of transplantation in children compared to adults.1
There are many reasons for the lower rate of donation in the paediatric population. Fortunately, child mortality is low, overall (ranging from 2.68 per 1000 inhabitants in infants aged less than 1 year to 0.07 per 1000 inhabitants in children aged 10–14 years) and in the paediatric intensive care unit (PICU) setting (approximately 2% of admissions to PICU).2 Infants and children considered potential donors require a complex and specific evaluation of each organ in addition to the evaluation involved in donor-recipient matching. In addition, controlled paediatric donation after circulatory death (pDCD) is still exceptional.3
Thus, additional efforts and strategies are needed to maximise the availability and benefit of transplantation for children and to ensure that the option of donation is offered to children and families as part of end-of-life care. Along these lines, the 2018–2022 strategic plan of the Organización Nacional de Trasplantes (National Transplant Organization, ONT) includes the objective of optimising paediatric donation and transplantation, for which a specific working group has been created through the collaboration of the Asociación Española de Pediatría (Spanish Association of Pediatrics, AEP) and the ONT (Appendix A). This group has developed a consensus document to facilitate the process of donation in children and infants and analysing the associated ethical dilemmas. The document specifically elaborates on the process of pDCD, including pDCD in special situations (severe central nervous system anomalies incompatible with life and children in palliative care). This article is a summary of the full document, which is available through the ONT and the AEP.4
MethodologyWe formed a group of experts selected by the ONT and the AEP. The project did not receive any external funding and participants reported having no conflicts of interest. The ONT coordinated the working group, held in-person meetings in its headquarters and facilitated a shared working platform.
The recommendations are based on current scientific evidence, documents published by international organizations and expert consensus taking into account the social reality and current law in Spain. Ethical and legal aspects were reviewed and discussed in depth with the collaboration of experts in bioethics of the ONT. Before the document was approved, it was subject to a public consultation process involving professionals affiliated to numerous scientific societies. The final version of this document has been endorsed and adopted by the Transplantation Commission of the Interterritorial Council of the National Health System and the executive board of the AEP.
Ethical principles in paediatric donationPaediatric donation is encompassed in end-of-life care and therefore recommendations have been issued to consider it both in case of brain death (BD) and after the decision for withdrawal/withholding life-sustaining treatment (WLST), when the possibility of donation following determination of death based on circulatory and respiratory parameters can be brought up, as established by Royal Decree 1723/2012.5,6
Paediatric donation involves shared decision-making with the family (family-centred care), and it is the parents that, based on the information received and their own values and beliefs, may consent to their child becoming a donor if they perceive it as something positive for the child and for themselves.
In Spain, parents and legal guardians are the sole parties authorised to decide whether a minor aged less than 18 years can become a donor, although 3 ethical principles must be applied: a) “do no harm” to the child; b) “substituted judgment”, by which the proxy decision-maker needs to answer the question “if the child was sufficiently mature, what would he or she elect?”, and c) the “best interests” of the child, which involve answering the question “do we want what would be best for our child?”.
Donation may be beneficial to the family by offering an option that could give some meaning to the loss of the child, who would leave a legacy of life for other people and that, in being an altruistic act, could counteract the tragedy of the premature death of a child.
If parents consider organ or tissue donation to fit their values and express it to be so, the care team and the transplant coordinator (TC) must integrate this decision in the care plan, providing a simple, clear and compassionate explanation of the details of the process.
From an ethical standpoint, the transition from WLST to obtaining consent for donation may appear complex and conflict-ridden, as care at the end of life may need to be prolonged to make donation possible. The care team will contact the TC early on and clearly explain to the family that the processes of WLST and of donation are independent and that end-of-life care will be provided in any case, even if donation is refused.
Evaluation of potential donorsIt entails a comprehensive evaluation to, on one hand, rule out the presence of diseases that could be potentially transmitted from donor to recipient (especially in potential donors with cancer or infectious disease) and, on the other, will assess the structure and function of organs that could be transplanted, always taking into account that one not being suitable does not mean that others could not be viable.
The details of this evaluation (comprehensive history taking, thorough physical examination and diagnostic tests), including an addendum on the subject of infection by coronavirus, are given in the full document of the working group4 and summarised in Table 1.
Process of evaluation of a potential organ and tissue donor.
| Comprehensive history | Personal history: chronic diseases, transfusions, vaccination history, epidemiological risks, contact with animals, travel to endemic regions |
| Family history | |
| Current disease: hypotension, pharmacotherapy, duration of asystole and cardiopulmonary resuscitation | |
| Physical examination | Full exam, including anthropometric measurements (height, weight and waist and chest circumference) |
| Matching | Blood typing and human leukocyte antigen (HLA) typing |
| Blood tests | Complete blood count, coagulation study, kidney, liver and pancreas function tests, blood gases |
| Serology | Human immunodeficiency virus (HIV). Hepatitis B, C or D virus. Human T-cell leukaemia virus type 1 or 2. Cytomegalovirus. Epstein-Barr virus (EBV) |
| Syphilis. Toxoplasma | |
| In specific cases: Trypanosoma cruzi and geographically restricted infections | |
| Microbiology | Blood culture, urine culture, throat swab culture |
| Cardiology | Electrocardiogram, echocardiogram |
| Imaging | Chest radiograph, abdominal ultrasound, consider abdominal/thoracic computed tomography |
| Fibreoptic bronchoscopy | Consider in potential lung donors |
Once the necessary information is collected, the paediatrician in charge will contact the TC, who will in turn decide which organs and tissues are suitable for transplantation.
What are the absolute contraindications to donation?There are actually very few, which are: positive test for detection of human immunodeficiency virus (HIV), human T-cell leukaemia virus (HTLV) type 1 or 2, untreated active systemic infection as cause of death, prion disease, disseminated hydatidosis or recent history of cyst resection, haematologic disease of unknown aetiology (e.g. aplastic anaemia) collagenosis and vasculitis, blood cancer or cancer with potential for metastasis, certain cancers of the CNS. The CT will be the person responsible for determining the presence of contraindications to donation in every case.
Are there specific contraindications in the paediatric population?In newborns, a gestational age of less than 34 weeks and a birth weight of less than 2 kg limit the viability of organs (due to immaturity and lack of recipients), although donation of heart valves can be considered in this group. In cases of inherited disease, chromosomal disorder, congenital error of metabolism or congenital anomaly, the TC will make an individualised assessment.
Diagnosis of brain death in infants and childrenThe diagnosis of BD is an absolute requisite for paediatric donation, one that is therefore specifically expressed in a Royal Decree in Spain (1723/2012).6 Spain and other countries have developed various guidelines on the subject.7 The document of the ONT-AEP provides a detailed explanation of the process for determining BD in children,4 of which we provide the key points:
- a)
Factors supporting diagnosis. Irreversible coma of unknown aetiology with clinical features or imaging findings consistent with devastating brain injury. Reversible causes of coma and apnoea must be ruled out, especially drugs known to depress the CNS.
- b)
Neurologic examination. It must be systematic and thorough, ensuring that the prerequisites detailed in Table 2 are met. Key findings include irreversible coma, absence of brainstem reflexes, lack of response to atropine administration and apnoea.
Table 2.Prerequisites for neurologic evaluation of children with suspected brain death.
Haemodynamic and metabolic stability Adequate oxygenation and ventilation Body temperature: > 35 °C in children < 2 years and > 32 °C in older children More than 24 h elapsed from the time of brain damage Absence of neuromuscular blockers Sufficient time elapsed for elimination of CNS depressant drugs Evidence of a cause of death - c)
Observation period. The length must be determined based on the type and severity of the damage and the tests performed (Table 3).
Table 3.Observation periods required for diagnosis of brain death in children per Royal Decree 1723/2012.
Exclusively clinical diagnosis Clinical diagnosis and one instrumental testa Preterm newborn (< 37 weeks) 48 h The observation period may be shortened as deemed appropriate by the clinician based on the findings of performed instrumental tests and may be omitted if a diagnostic test unequivocally proves absence of cerebral blood flow Newborn (37 weeks’ gestation to 20 days post birth) 24 h CHILD (age 30 days-24 months) 12 h CHILD aged more than 2 years with devastating brain injury 6 h The established observation periods may be shortened or even omitted as deemed appropriate by the clinician based on instrumental diagnostic tests performed in accordance to the protocols established for adults CHILD aged more than 2 years with anoxic brain injury 24 h In case of suspected or known exposure to drugs known to have a depressant effect, the observation period should be prolonged as deemed appropriate by the clinician.
- d)
Confirmatory instrumental tests of brain death. They include tests that assess neuronal function (electroencephalogram and evoked potentials) and cerebral blood flow (arteriography, digital subtraction angiography, cerebral scintigraphy, transcranial Doppler ultrasound, multidetector computed tomography [CT] angiography of the head and magnetic resonance angiography). One or several of these tests must be performed when the neurologic evaluation is inconclusive or for the purpose of shortening the observation period. Each of these techniques has limitations when it comes to children, so individualised selection of imaging tests is recommended, in addition to, if possible, the combination of 2 or more of these tests.
The management of paediatric organ or tissue donors include interventions aimed at maintaining organ perfusion to ensure viability and functionality after transplantation. To this end, these patients should be managed in a PICU or a neonatal intensive care unit (NICU), as these units have the necessary staff and technological resources.
In the process of BD, physiological changes take place that may alter perfusion and organ function, and these must be known, detected and managed. Chief among them are the so-called “catecholamine storm”, vasoplegia, hypotension, diabetes insipidus, arrythmia, the release of inflammatory mediators, electrolyte imbalance, acid-base imbalance and hypothermia. Respiratory support is essential to maintain oxygenation and ventilation, striving to avoid the potential lung damage associated to mechanical ventilation. Haematologic abnormalities (anaemia, coagulation abnormalities) and renal and electrolyte abnormalities must be managed, especially those associated with neurogenic diabetes insipidus. The prevention and treatment of infection are essential to avoid transmission to the recipient.
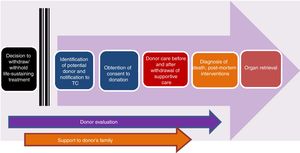
Controlled paediatric donation after circulatory deathThe reduced size of the pool of potentially suitable paediatric donors has spurred the development of strategies to increase the possibility of transplantation in children, such as surgical reduction or splitting of organs or living donor transplantation, among others. In this context, controlled pDCD, understood as donation by individuals deceased following an anticipated cardiac arrest, the WLST decision-making process and discontinuation of life support (Maastricht category III),3,4,8 is particularly important (Fig. 1). Table 4 summarises the differences between pDCD and donation after BD.
Stages of the process of controlled paediatric donation after circulatory death (adapted from Thuong et al.8).
Controlled donation after circulatory death vs donation after brain death.
| Controlled donation after circulatory death | Donation after brain death | |
|---|---|---|
| Diagnosis of death | Circulatory criteria | Neurologic criteria |
| Who diagnoses death? | A physician not involved in the donation and transplantation processes | Three physicians not involved in the donation and transplantation processes |
| When is death diagnosed? | After 5 min of continuous asystole following withdrawal of life support | On completion of the established protocol for diagnosis |
| When is donation discussed with the family? | Before the diagnosis of death | Usually after diagnosis of brain death |
| Warm ischaemia time | Occurs. May compromise organ viability | Does not occur |
| Effectiveness of donation | Fewer organs available for retrieval and difficulty predicting whether asystole will occur within the established time frame | In principle, more organs can be recovered |
The potential paediatric donors after circulatory death are children for who the care team and parents have agreed on WLST, have no contraindications and in who cardiac arrest is expected to happen following withdrawal of life support in a time frame compatible with donation. The pathological condition leading to the decision to WLST is usually neurologic with a known lethal outcome but in which progression to BD is not anticipated, or any other in which curative treatment has failed and the prognosis is bleak. Soon after the decision has been made to forgo life-sustaining treatment, the care team must notify the case to the TC to proceed to the evaluation of the patient and the donation conversation with the family.
Who should talk to the family about donation?It is recommended that the TC and the paediatrician in charge of the patient have the donation conversation with the family. Both ought to explore in a gradual and tactful manner whether organ and tissue donation is consistent with the values of the child and the family. If it is, the family must be informed of the need to transfer the patient to the operating room for discontinuation of life support, the way in which family members will be able to bid farewell to the child, the possibility that the child will die in a time frame incompatible with the pDCD protocol, in which case organs could not be recovered, that the patient will need to return to the PICU (or another inpatient unit) for end-of-life care, the possibility of donating specific organs or tissues and, in some cases, the need for ante-mortem interventions and what these would entail.
Where and how should life-sustaining treatment be withdrawn?Once the decision is made for WLST and the family has provided informed consent, the withdrawal of life-sustaining treatment (following hospital protocol with the approval of the clinical ethics committee) is usually performed in the operating room with the purpose of shortening the ischaemia time. It is recommended that WLST is carried out by the paediatrics care team and an anaesthesiologist, ensuring delivery of appropriate sedation and analgesia and allowing the parents to be present. The surgical team should be on standby and ready to start surgery once the TC calls for it.
If death does not take place in a time frame compatible with pDCD after withdrawal of life-sustaining treatment,6 palliative care will continue until the patient’s death in the previously selected setting (PICU, NICU or palliative care room), keeping in mind that the patient might still become a tissue donor after death.
How is death diagnosed based on circulatory and respiratory criteria?Based on the directives of Royal Decree 1723/2012,6 the diagnosis of death on the grounds of circulatory and respiratory criteria is based on the presence of unequivocal evidence of absence of spontaneous circulation and respiration for a period of at least 5 min. The absence of circulation must be demonstrated with at least one of the following: asystole in the continuous electrocardiogram, absence of blood flow evinced through invasive blood pressure monitoring, or absence of aortic blood flow in the echocardiogram. The paediatrician in charge of the patient (who must not be involved in the donation process) is responsible for certifying the death of the child.
In the special situation of children connected to an extracorporeal membrane oxygenation (ECMO) system in who this intervention is deemed to be futile, donation may be considered after the decision to withdraw this support, which will lead to the end of circulation and breathing.
What are ante-mortem interventions, and what are their implications?These are interventions performed before death with the aim of facilitating pDCD, including transfer to the operating room, administration of drugs and performance of certain invasive techniques (such as placement of central access lines) to improve organ viability. Performance of these interventions requires specific informed consent, explaining to parents what they entail, the associated risks and that their purpose is to improve organ viability for transplantation.
What are the techniques used to recover organs in pDCD?Organs should be recovered as soon as possible (“super rapid” retrieval), although there is a growing use of normothermic regional perfusion (NRP) techniques based on the use of ECMO devices, which allow improved in situ preservation or organs with oxygenated blood. Normothermic regional perfusion regenerates tissues damaged by ischaemia, allowing the functional evaluation of organs before procurement and changing urgent retrieval to a pseudo-elective procedure similar to retrieval in donation after BD.
What are the ischaemia times?3,4The total warm ischaemia time is the time elapsed from WLST to initiation of in situ organ preservation (NRP) or organ retrieval. The actual or functional warm ischaemia time is the time elapsed from the beginning of significant hypotension following WLST to initiation of in situ organ preservation or organ retrieval. The in situ preservation time is the time elapsed from initiation of NRP to initiation of surgical retrieval. The cold ischaemia time is the time elapsed from initiation of chilled perfusion of the organ to the transplantation surgery.
In adults, the maximum functional warm ischaemia time allowed is 30 min for the liver and 60 min for the kidneys and lungs. The data on the maximum ischaemia times in pDCD are still inconclusive.3,4
What are the ethical considerations brought up by pDCD?Paediatric donation after circulatory death involves modification of the standard care delivered before the death of the donor, which raises a series of ethical dilemmas.3,4 One of the most important controversies concerns the validity of proxy consent to interventions in the donor (ante-mortem interventions) for the benefit of the recipient. Thus, proxy consent requires the fulfilment of the following conditions: a) the risks to the donor are minimal and similar to the risks experienced by children undergoing treatment with curative intent; b) parents are informed and given sufficient time to assimilate the information about the procedure and the opportunity to discuss their concerns; c) specific informed consent will be obtained for each ante-mortem intervention that needs to be performed, and d) parents consider donation as an expression of the belief that their child (if the child were able to communicate) and themselves would wish for the organs to be transplanted.
When it comes to post-mortem interventions (before organ retrieval), NRP should be used, avoiding the potential risk of re-establishing cerebral blood flow, which would invalidate the diagnosis of death. To prevent this, the abdominal aorta is blocked through the insertion of a balloon or surgical clamping.
Another source of controversy is the conflict of values that may be experienced by health care professionals involved in the care of potential donors, as in the case of pDCD the child, already receiving paediatric palliative care (PPC), is subjected to interventions that will not provide the patient any clinical benefit. For this reason, the working group recommends that both parents and providers are given a clear and detailed explanation of every step in the process of pDCD and of the role of each member of the team.
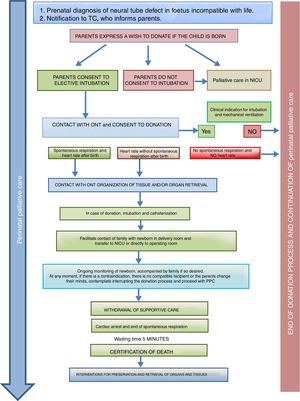
Donation in special situationsThe ONT-AEP document includes a novel section on donation in 2 special situations. On one hand, newborns with neural tube defects incompatible with life in who PPC starts at the time of prenatal diagnosis and continues with labour and delivery care plans, emotional support and counselling for the family, shared decision-making during the life of the child and the grieving process. Such perinatal PPC must include consideration of organ and tissue donation and the activation in conjunction with the TC of the pDCD protocol, taking into account its complexity and any technical challenges (Fig. 2).
Process of organ and tissue donation in anencephalic newborns.4
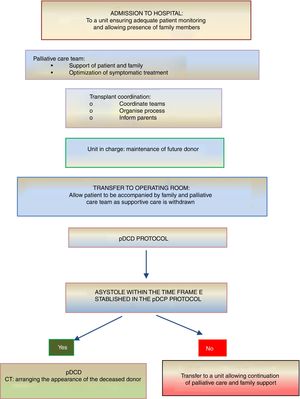
On the other hand, donation in children receiving PPC at home is another novel situation that is controversial and requires a delicate approach. If the parents or legal guardians and possibly also the child (if intellectually and emotionally capable) make their wish to donate express as part of the care plan and reiterate this will throughout the disease, donation may be an option in the absence of contraindications. In this case, the decision to withdraw life-sustaining treatment will always be made beforehand exclusively by the patient, parents and paediatrician in charge. The subsequent evaluation of the suitability of the patient for donation of organs and tissues will be the responsibility of the TC and will occur following and be independent of the decision of WLST. If the evaluation by the TC is favourable and the parents (and, if possible, the child) reiterate a desire to donate, the child will be transferred to hospital for the donation procedure, which will be similar to the process undergone by an inpatient. In all cases, patients and families will offer psychological and spiritual support fitting their preferences, as well as end-of-life PPC (Fig. 3).
Summary of the process of pDCD in children receiving palliative care at home.4,5
Routine consideration of donation in children and newborns when their deaths occur in circumstances compatible with donation is an ethical standard that should become a clinical practice standard. This practice does not only increase the availability of organs for transplantation, but also promotes an integrated family- and patient-centred care approach.
AcknowledgmentsWe thank the group of experts in bioethics of the ONT: Diego Gracia Guillén, Alicia Pérez Blanco, Teresa Aldabó Pallas, Josep María Busquets Font, Sebastián Iribarren Diarasarri, José Luis López del Moral Echevarría, Fernando Martínez Soba, Montserrat Nieto Moro, José Miguel Pérez Villares, José Ignacio Sánchez Miret, José Antonio Seoane Rodríguez, Alejandro Toledo Noguera.
Joan Balcells Ramírez, Rebeca Bajo Rodilla, Dorotea Blanco Bravo, Carmen Camarena Grande, Isabel Caro Portela, Sonia Caserío Carbonero, Beatriz Domínguez-Gil González, Raquel Escrig Fernández, Belén Estebánez Montiel, Juan Galán Torres, Fernando Gómez Sáez, Antonio Gordillo Brenes, José Luis López del Moral Echevarría, Álvaro Navarro Mingorance, Montserrat Nieto Moro, Alicia Pérez Blanco, Ruth Pérez Montejano, Teresa Pont Castellana, Antonio Rodríguez Núñez and Cristina Vidal Tobar.