The neutrophil-to-lymphocyte ratio (NLR) is an inflammatory biomarker that is easily calculated with data from the differential white blood cell count. The aim of our study was to analyse the role of the NLR in the detection of negative appendectomies and to compare its usefulness with other clinical, sonographic and laboratory factors previously described.
MethodsWe conducted a retrospective study in patients aged less than 16 years who underwent appendectomy in our hospital between 2017 and 2020. We divided patients into 2 groups based on appendiceal histological findings: NA group (negative appendicitis: absence of appendiceal inflammation) and PA group (positive appendicitis: presence of inflammation in any layer of the appendiceal wall). We analysed demographic, clinical, sonographic and laboratory characteristics.
ResultsWe included a total of 1269 patients, 1244 in the PA group and 25 in the NA group, with no differences between groups in demographic characteristics. The proportion of patients that presented with nausea and vomiting was significantly smaller in the NA group compared to the PA group (P < .001), and there were no other differences in symptoms. The appendiceal diameter on ultrasound was significantly smaller in the NA group (8.1 ± 2.1 vs. 9.7 ± 2.8 mm; P < .001). The white blood cell and neutrophil counts and the NLR were significantly higher in the PA group (P < .001), as was the level of C-reactive protein (18.6 vs. 2.6; P = .005). The ROC curve analysis revealed that the NLR was the parameter with the highest AUC (0.879) for the diagnosis of negative appendicitis, with a cut-off point of 2.65 for a maximum sensitivity of 84.2% and specificity of 83.8%.
ConclusionThe NLR is the preoperative parameter that best discriminates patients without acute appendicitis. Values of less than 2.65 should make clinicians contemplate diagnoses other than appendicitis.
El índice neutrófilo-linfocito (INL) es un biomarcador inflamatorio fácilmente calculable a partir del recuento diferencial de leucocitos. El objetivo de este estudio es analizar el papel del INL en la detección de apendicetomías negativas, y comparar su utilidad con otros factores clínicos, ecográficos y de laboratorio previamente descritos.
MétodosEstudio retrospectivo en pacientes menores de 16 años con sospecha de apendicitis aguda intervenidos en nuestra institución entre 2017–2020, que fueron divididos en 2 grupos según hallazgos histológicos apendiculares: Grupo AN: apendicitis negativa; ausencia de inflamación y grupo AP: apendicitis positiva; presencia de inflamación en la pared apendicular. Se analizaron las características demográficas, clínicas, ecográficas y de laboratorio.
ResultadosSe incluyeron 1.269 pacientes (1.244 en el grupo AP; 25 en el grupo AN), sin diferencias demográficas entre ellos. Los pacientes del grupo AN presentaron un porcentaje significativamente menor de náuseas y vómitos en comparación con el grupo AP (p < 0,001) y menor diámetro ecográfico apendicular (8,1 ± 2,1 vs. 9,7 ± 2,8 mm; p < 0,001). Los recuentos de leucocitos, neutrófilos e INL fueron significativamente superiores en el grupo AP (p < 0,001), así como la proteína C reactiva (18,6 vs. 2,6; p = 0,005). El análisis mediante curva ROC mostró que el INL fue el parámetro con mayor AUC (0,879) para el diagnóstico de apendicitis negativa, con un punto de corte de 2,65 con una sensibilidad del 84,2% y una especificidad del 83,8% máximas.
ConclusionesEl INL es el parámetro preoperatorio que mejor distingue a los pacientes sin apendicitis aguda. Los valores inferiores a 2,65 deben hacernos sospechar otra causa diferente a la apendicitis.
Acute appendicitis (AA) is the most common surgical emergency in the paediatric population.1 The natural progression from acute inflammation to perforation and peritonitis typically occurs over a period of a few days.2 Early and accurate diagnosis of AA is critical in preventing appendiceal perforation, which can lead to severe morbidity or death if untreated.3 Therefore, appendicitis should ideally be diagnosed and treated before the appendix ruptures, while limiting the frequency of removal of normal appendices, which is known as the negative appendectomy (NA) rate.4 In the past, several studies have justified the high NA rate based on the need to prevent appendiceal perforation. It was assumed that the morbidity associated with a NA was not severe enough compared to the risk of appendiceal perforation.5
However, in recent years it has been reported that NA is associated with a longer length of stay (LOS), increased morbidity and higher costs compared to appendectomy in patients with nonperforated appendicitis.6 For this reason, numerous studies have attempted to improve the accuracy of the diagnosis of AA and decrease the NA rate through the application of scoring systems and imaging techniques.7,8 Laboratory parameters such as the white blood cell (WBC) count and the level of C-reactive protein (CRP) are elevated in multiple inflammatory conditions, including AA. Attempts have been made to establish cut-off points to predict the preoperative diagnosis of AA, with contradictory results.9,10
The neutrophil-to-lymphocyte ratio (NLR) is an inflammatory biomarker that is easily calculated with data from the WBC differential. Recent studies have described its role as a predictor of peritonitis and postoperative intra-abdominal abscess in children.11,12 However, its usefulness in predicting negative appendectomy has not been described to date. The aim of our study was to analyse the usefulness of the NLR in the discrimination of negative appendectomies in children, comparing it with other, previously applied clinical, sonographic and laboratory parameters.
MethodsWe conducted a retrospective study in patients aged less than 16 years with suspected acute appendicitis who underwent surgical treatment in our hospital between January 2017 and December 2020. We divided patients into 2 groups based on the histological findings of the examination of the surgical specimen: NA group (negative appendicitis: absence of inflammation in the caecal appendix) and PA group (positive appendicitis: presence of inflammation in any layer of the appendiceal wall). The PA group included patients with limited inflammation of the appendiceal mucosa, transmural inflammation and wall perforation with signs of inflammation. The same 2 pathologists performed the histological examination of the caecal appendix in every case. We excluded patients who underwent incidental appendectomies as part of another procedure or delayed or interval appendectomies after nonoperative management for perforated appendicitis. In all cases, the diagnosis was confirmed by an abdominal ultrasound examination.
We analysed demographic characteristics, clinical features, time elapsed from onset, sonographic findings and laboratory test results. When it came to clinical features, we collected data on the presence of right iliac fossa pain, fever (defined as a temperature ≥ 37.5 °C), nausea, vomiting, diarrhoea, constipation, dysuria, anorexia and upper airway symptoms. The sonographic features included the appendix diameter, presence of periappendiceal inflammation, peritoneal or pelvic free fluid, enlargement of adjacent lymph nodes and appendicolith. We obtained data on laboratory parameters from the blood tests performed in the emergency department on the patient’s arrival, which included a complete blood count with differential (total leukocyte count, neutrophils, lymphocytes, monocytes, basophils and eosinophils), blood chemistry panel (electrolyte panel, glucose, urea, fibrinogen), and CRP levels. We calculated the NLR by dividing the total neutrophil count by the total lymphocyte count. The study protocol conformed to the principles of the 1975 Declaration of Helsinki and was approved by our institutional review board and the ethics committee of the hospital. Written informed consent was not required due to the retrospective nature of the study and the anonymous collection of laboratory data, in adherence with institutional guidelines.
We collected data with the software Microsoft Excel version 2010 (Redmond, WA, USA), and analysed it with the package SPSS Statistics version 22 (Chicago, IL, USA). The distribution of quantitative variables was assessed by means of the Kolmogorov-Smirnov and Shapiro-Wilk tests. We expressed normally distributed continuous data as mean and standard deviation (SD), and compared them with the Student t-test for independent samples. In the case of non-normally distributed continuous data, the expressed them as median and interquartile range (IQR) and made comparisons with the Mann-Whitney U test was used. Categorical variables were expressed as frequency (n) and percentage (%), and analysed with the chi square test or the Fisher exact test if the former did not apply. We performed a binomial logistic regression analysis to determine which clinical and sonographic features were associated with positive appendicitis versus negative appendicitis. We calculated odds ratios (ORs) with the corresponding 95% confidence intervals. Subsequently, we performed a multivariate analysis that included the variables identified in the bivariate analysis. For continuous variables, we generated receiver operating characteristic (ROC) curves. We calculated the area under the curve (AUC) and identified appropriate cut-off points to determine the sensitivity and specificity (Youden index).13 All statistical calculations were performed with 2 tails and statistical significance was defined as a p-value of less than 0.05.
ResultsA total of 1269 patients (793 male, 476 female) were included, with a median age at diagnosis of 10.5 years (IQR, 8.1–12.9). They were divided according to appendiceal histological findings (1244 in the PA group and 25 in the NA group), with no differences in demographic characteristics between the 2 groups. When it came to the clinical characteristics, a significantly smaller percentage of the NA group presented with nausea and vomiting compared to the PA group (P < .001), with no differences in the rest of the presenting symptoms. As for the sonographic findings, the mean appendiceal diameter was 8.1 (SD, 2.1) in the NA group, significantly smaller than in PA group (9.7 ± 2.8; P < .001). The presence of periappendiceal inflammation was also significantly lower in patients that did not have appendicitis compared to the PA group (56% vs 83.6%; P < .001). We did not find differences in the rest of the sonographic findings. Table 1 summarises the demographic, clinical and sonographic characteristics of patients in each group. The multivariate analysis did not show any correlation between the variables found to be positively associated in the bivariate analysis: nausea (P = .075), vomiting (P = .218) and periappendiceal inflammation (P = .063).
Demographic, clinical and ultrasound findings in both groups.
| PA group (n = 1244) | NA group (n = 25) | OR (95% CI) | P | |
|---|---|---|---|---|
| Sex; n (%) | 1.11 (.50−2.50) | .795 | ||
| • Male | 778 (62.5%) | 15 (60%) | ||
| • Female | 466 (37.5%) | 10 (40%) | ||
| Age (years); median (IQR) | 10.5 (8.1−12.8) | 11.7 (9.8−14) | – | .032 |
| Associated symptoms; n (%) | ||||
| • RIF pain | 951 (76.4%) | 19 (76%) | 1.02 (.41−2.59) | .989 |
| • Fever | 376 (30.2%) | 8 (32%) | .92 (.39−2.15) | .848 |
| • Nausea | 844 (67.8%) | 6 (24%) | 6.68 (2.65−16.86) | <.001 |
| • Vomiting | 735 (59.1%) | 5 (20%) | 5.78 (2.15−15.49) | <.001 |
| • Diarrhoea | 156 (12.5%) | 3 (12%) | 1.05 (.31−3.55) | .936 |
| • Constipation | 55 (4.4%) | 1 (4%) | 1.11 (.15−8.35) | .919 |
| • Dysuria | 93 (7.5%) | 1 (4%) | 1.93 (.26−14.5) | .510 |
| • Anorexia | 480 (38.6%) | 7 (28%) | 1.62 (.67−3.90) | .280 |
| • Upper airway symptoms | 68 (5.5%) | 2 (8%) | .66 (.15−2.88) | .583 |
| Time from symptoms onset (hours); median (IQR) | 24 (12−36) | 24 (16.5−48) | – | .263 |
| US appendiceal diameter (mm); mean ± SD | 9.7 ± 2.8 | 8.1 ± 2.1 | – | .002 |
| Sonographic findings; n (%) | ||||
| • Periappendiceal inflammation | 1040 (83.6%) | 11 (56%) | 4.0 (1.79−8.95) | <.001 |
| • Peritoneal/pelvic free fluid | 812 (65.3%) | 17 (68%) | .88 (.37−2.07) | .777 |
| • Adjacent lymph node enlargement | 385 (30.9%) | 8 (33.3%) | .95 (.41−2.23) | .910 |
| • Appendicolith | 342 (27.5%) | 2 (8%) | 4.36 (1.02−18.6) | .030 |
CI, confidence interval; IQR, interquartile range; NA, negative appendicitis; PA, positive appendicitis; OR, odds ratio; RIF, right iliac fossa; SD, standard deviation; US, ultrasound.
The analysis of laboratory parameters revealed significant differences in WBC counts. The leukocyte and neutrophil counts and the NLR were significantly higher in the PA group (P < .001). In contrast, patients in the NA group had statistically higher lymphocyte, monocyte, basophil and eosinophil counts compared to those in the PA group, all statistically significant differences. The median CRP level in the NA group was 2.6, significantly lower compared to the PA group (18.6; P = .005). There were no statistically significant differences in the platelet count or the fibrinogen, glucose, urea and electrolyte levels. Table 2 compares the laboratory findings at admission in the 2 groups.
Comparison of laboratory values at admission between the two groups.
| PA group | NA group | P | |
|---|---|---|---|
| Leukocytes (103/µL) | 15 395 (12 252−18 300) | 7400 (6425−9150) | <.001 |
| Neutrophils (103/µL) | 12 337 (9299−15 366) | 4181 (3185−6320) | <.001 |
| Lymphocytes (103/µL) | 1621 (1120−2278) | 2111 (1629−2927) | .001 |
| Monocytes (103/µL) | 824.5 (576.2−1115.8) | 572 (500−838) | .003 |
| Eosinophils (103/µL) | 72.1 (25−182) | 207 (44.4−275) | .009 |
| Basophils (103/µL) | 34 (18.8−52.6) | 44.5 (36.5−66.3) | .005 |
| NLR | 7.6 (4.4−12.4) | 1.9 (1.1−3.0) | <.001 |
| Platelets (/µL) | 279 000 (237 000−323 000) | 286 000 (236 000−328 000) | .663 |
| CRP (mg/L) | 18.6 (5.3−53.7) | 2.9 (.8−19.3) | .005 |
| Glucose (mg/dL) | 97 (87−110) | 90 (85−100) | .065 |
| Urea (mg/dL) | 27 (22−32) | 30 (22−33) | .984 |
| Fibrinogen (mg/dL) | 409 (323−519) | 346 (281−455) | .051 |
| Electrolytes (mmol/L) | |||
| • Sodium | 137.2 (135.8−139) | 139 (137.1−140.5) | .062 |
| • Potassium | 4 (3.8−4.2) | 4.1 (3.8−4.3) | .585 |
| • Chloride | 103 (101−106) | 105 (103−107) | .110 |
CRP, C-reactive protein; NLR, neutrophil to lymphocyte ratio; NA, negative appendicitis; PA, positive appendicitis.
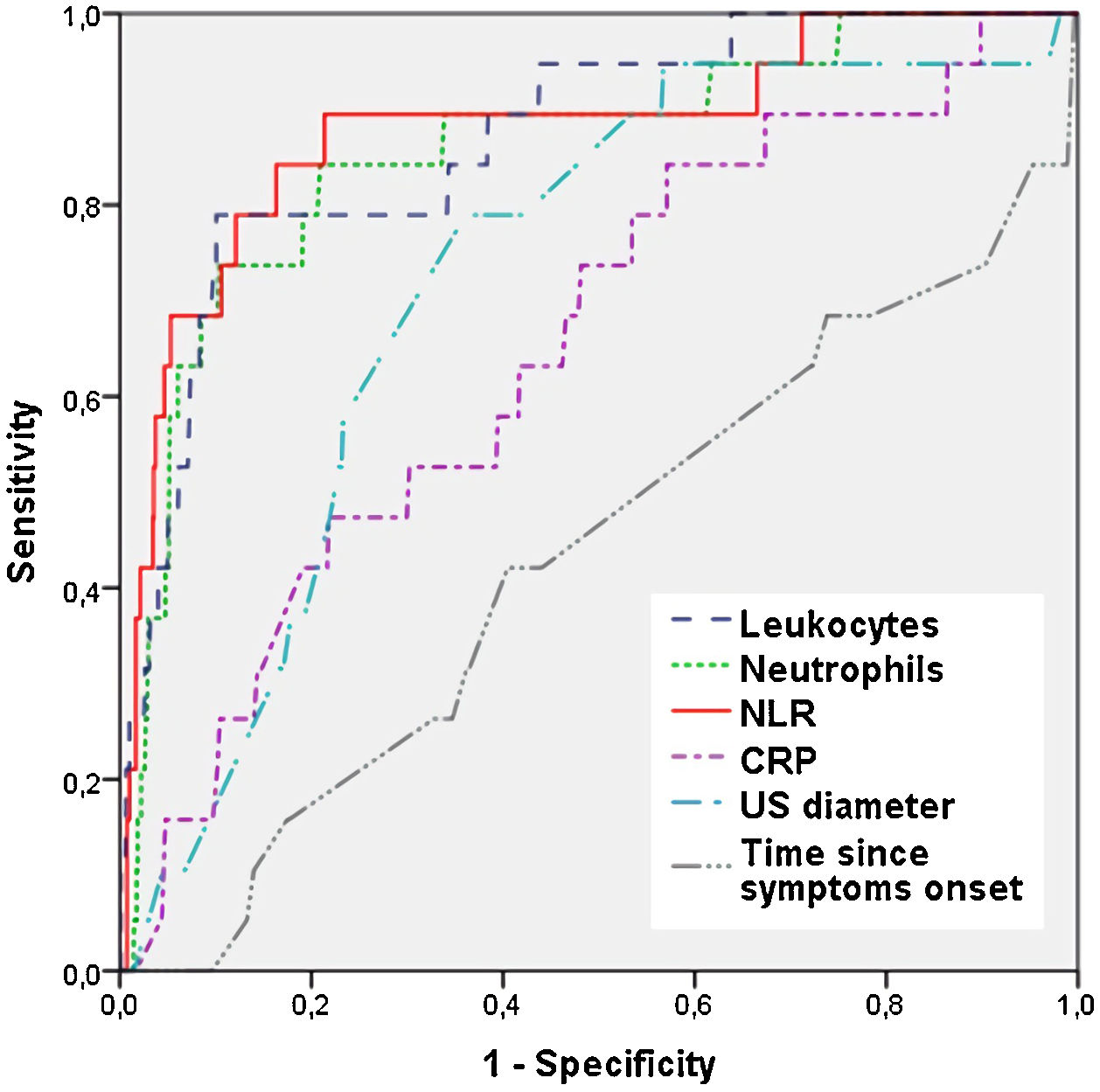
The sensitivity and specificity analysis of continuous variables using ROC curves (Table 3) showed that the NLR was the parameter with the highest AUC (0.879) for the diagnosis of negative appendicitis. A cut-off point of 2.65 was established to achieve the maximum sensitivity (84.2%) and specificity (83.8%), with a positive predictive value of 95.7%. The NLR had a significantly higher AUC compared to those of the neutrophil count (0.871), leukocyte count (0.857) and sonographic appendiceal diameter (0.723), differences that were statistically significant (P < .001). Fig. 1 shows the ROC curves for laboratory tests, sonographic diameter and time from onset for diagnosis of negative appendicitis.
Area under the ROC curve for negative appendectomy.
| AUC (95% CI) | Cut-off point | Sensitivity | Specificity | P | |
|---|---|---|---|---|---|
| NLR | .879 (.788−.971) | 2.65 | 84.2% | 83.8% | <.001 |
| Neutrophils (103/µL) | .871 (.790−.951) | 7600 | 78.9% | 89.6% | <.001 |
| Leukocytes (103/µL) | .857 (.765−.949) | 9200 | 73.7% | 89.8% | <.001 |
| US diameter (mm) | .723 (.623−.823) | 8.0 | 78.9% | 63.9% | .003 |
| CRP (mg/L) | .652 (.532−.772) | 24.5 | 84.2% | 42.7% | .023 |
| Time from onset (hours) | .433 (.294−.573) | 18 | 42.1% | 59.5% | .320 |
AUC, area under the curve; CI, confidence interval; CRP, C-reactive protein; NLR, neutrophil to lymphocyte ratio; US, ultrasound.
We considered an AUC > .600 and a P < .05 statistically significant.
This retrospective single-centre study analysed data for 1269 patients who underwent appendectomy in the last 4 years in our hospital with the aim of establishing the role of the NLR as a predictor of negative appendectomy, and comparing it with previously used clinical features, sonographic findings and laboratory parameters. Based on our results, the NLR can be considered the preoperative parameter with the highest sensitivity and specificity for predicting the absence of appendicitis in clinically suspected cases.
Historically, surgical dogma justified a NA rate as high as 15% to 25% to prevent negative outcomes such as perforation, peritonitis, abscess or prolonged hospitalization.14 In children, the acceptable rate has been even higher, perhaps due to the difficulty of obtaining an accurate clinical history and physical examination in young patients. However, the belief that the NA rate may be inversely related to the rate of perforated appendicitis has since been abandoned.5 Reported NA rates have decreased substantially over the last decade with the advent of advanced imaging modalities, such as ultrasound and cross-sectional imaging.4,15
The current literature reveals NA rates ranging from 1% to 10%.16,17 This variation may be explained by differences in the definitions of appendicitis and negative appendectomy used by institutions to calculate the NA rate, as most of these studies do not provide clear definitions in relation to pathology findings. While transmural inflammation of the appendix is the most common definition, some institutions, our hospital among them, include mucosal inflammation/ulceration as a sign of early appendicitis. The NA rate in our study was 2%, similar to recent reports in the paediatric population.9
Despite its ubiquity, acute appendicitis can be much more difficult to diagnose in children compared to adults because the findings of the history and physical examination in children may not be typical, and it may also be difficult to obtain these findings in an uncooperative young child.18,19 After reviewing all NA cases in our hospital in the past 4 years, we found clinical variables equally unhelpful in identifying false-positives. In our study, a higher percentage of patients in the PA group presented with nausea and vomiting compared to the NA group, although there were no significant differences in other symptoms. Therefore, it is necessary to use other tests to guide the diagnosis of appendicitis in children.
The abdominal ultrasound, the imaging gold standard in children, can also be less informative than in adult patients due to difficulties in its performance. Moreover, since ultrasound is an operator-dependent technique, the sensitivity and specificity for the diagnosis of appendicitis depends on the experience of the radiologist with paediatric patients.20 In our hospital, ultrasound examinations are performed in all children with suspected appendicitis. The appendiceal diameter was significantly larger in the PA group (9.7 mm), which is due to progressive inflammation of the appendiceal wall, causing an increase in diameter starting from the tip towards the base. A cut-off point of 8 mm allows identification of negative appendicitis with a sensitivity of 80%, which was consistent with previous studies.20,21 The presence of periappendiceal inflammation is the other most frequent ultrasound feature observed in patients with AA. However, in the multivariate analysis it did not prove useful in identifying patients found not to have appendicitis based on histological appendiceal findings, which can be partly explained by the low negative appendicitis rate in our centre.
On the other hand, laboratory parameters are easily determined, unlike imaging, do not require expertise for their obtention or interpretation, and do not add to the financial burden on the patient or hospital.22 Rather than using laboratory parameters as dichotomous values (present or absent, based on an established cut-off point), we chose to treat them as continuous variables to assess their performance as we changed the discrimination threshold. However, the relative scarcity of NA patients (25 out of 1269 appendectomies) led to ROC curves that were somewhat irregular.
Our results indicate that the NLR performs well as a continuous variable, with an AUC of 0.879, a sensitivity of 84.2% and a specificity of 83.8% for a cut-off point of 2.65. It is the parameter that identifies patients without appendicitis most accurately, followed by the neutrophil count (AUC = 0.871) and the leucocyte count (AUC = 0.857). Goodman et al. first proposed that the NLR was more useful than total leukocyte count in the diagnosis of AA.23 In subsequent years, studies have been conducted to assess the correlation between the NLR and the severity of AA, treatment planning, and complications.12,24,25 However, the role of NLR as a predictor of negative appendectomy in children has not been described to date, so our study constitutes a first exploration of this area. There is evidence that the neutrophil count is more useful than the leucocyte count both for the diagnosis of AA and in grading simple and complicated appendicitis.26 Nevertheless, NLR increases diagnostic accuracy with respect to neutrophil and leukocyte counts because the immune response triggered by infectious, ischaemic or inflammatory factors leads to an increase in neutrophil count mediated by growth factors on haematopoietic stem cells, and a decrease in lymphocyte count due to apoptosis mediated by tumour necrosis factor α (TNF-α).27 In this context, the NLR is also an earlier marker of the acute phase response compared to CRP, as pooled neutrophils in bone marrow respond more rapidly to acute inflammation. C-reactive protein, in contrast, must be synthesized in the liver in response to interleukin and pro-cytokine pathway activation (IL-6), which results in a time lag.28 Furthermore, compared to CRP, the NLR requires only a simple test that does not add to the expense on the patient and can be easily calculated using parameters that are included in the complete blood count.
The limitations of this study derive from its single-centre and retrospective design. In addition, due to the low frequency of NA, there was a large difference in the sizes of the 2 groups. Notwithstanding, we found significant differences in the laboratory parameters of both groups that would allow the identification of patients in whom negative appendectomies should be avoided, as these are unnecessary operations that constitute a significant burden for both patients and the health care system.
ConclusionThe NLR is the preoperative parameter that best discriminates patients without acute appendicitis. Values of less than 2.65 should lead clinicians to contemplate diseases other than appendicitis. It is a simple and inexpensive screening tool that should be taken into account to prevent negative appendectomies.
FundingThis project did not receive any specific grant from funding agencies in the public, private, or not-for-profit sectors.