There is still controversy on the relationship between idiopathic premature adrenarche (IPA) and a history of small for gestational age, as well as the concomitant presence of obesity and other metabolic disturbances. An attempt is made to study these potential associations in a cohort of girls with IPA from our hospital.
Patients and methodsA descriptive cross-sectional study was conducted that included girls with a diagnosis of IPA from the Paediatric Department of the Fundación Jiménez Díaz (Madrid, Spain) between January 2007 and May 2015. A record was made of family and personal history with perinatal data, as well as anthropometric data and biochemical values at the time of diagnosis.
ResultsOut of a total of 76 girls with IPA, 2.7% had a history of small for gestational age. When body mass index was analysed according to modified criteria of WHO 2007/Cole 2000, 11.8% were overweight, and 11.8% were obese at diagnosis. Using the criteria set by the Spanish Ministry of Health, 6.6% were overweight and 18.4% obese, with 21.2% of the girls being insulin resistance, and 13.95% having dyslipidaemia. None of them had hypertension. From a comparative analysis between normal and overweight and obesity IPA girls, the latter had significantly higher levels of triglycerides and insulin, a higher HOMA index, and lower levels of HDL cholesterol.
ConclusionsIPA girls included in the study do not have a higher prevalence of small for gestational age compared to the general population. Prevalence of overweight and obesity in girls with IPA is not higher than the prevalence in the normal population.
Hasta la fecha hay datos contradictorios sobre la relación entre adrenarquia prematura idiopática (API) y el antecedente de pequeño para edad gestacional así como con la presencia de obesidad y otras alteraciones metabólicas. Es nuestra intención estudiar esa posible asociación en una cohorte de niñas con API de nuestro hospital.
Pacientes y métodosEstudio descriptivo transversal que incluyó a niñas diagnosticadas de API en el servicio de Pediatría de la Fundación Jiménez Díaz entre enero de 2007 y mayo de 2015. Se recogieron datos sobre antecedentes familiares, antecedentes personales que incluían datos perinatales así como datos antropométricos y datos bioquímicos al diagnóstico.
ResultadosDel total de 76 niñas con API, un 2,7% presentaba antecedente de pequeño para edad gestacional. Utilizando la clasificación del índice de masa corporal según criterios modificados de OMS 2007/Cole 2000, un 11,8% tenían sobrepeso y un 11,8% obesidad al diagnóstico. Según los criterios del Ministerio de Sanidad, un 6,6% presentaban sobrepeso y un 18,4% obesidad. Un 21,2% evidenciaron insulinorresistencia y un 13,95% presentaban dislipidemia. Ninguna de las pacientes cumplía criterios de hipertensión arterial. En el análisis comparativo entre niñas con API que presentaban normopeso frente a las que tenían sobrepeso y obesidad, las segundas presentaban niveles significativamente más elevados de triglicéridos e insulina y más bajos de colesterol HDL.
ConclusionesLas niñas con API estudiadas no presentan mayor porcentaje de pequeño para edad gestacional que la población general. La prevalencia de sobrepeso y obesidad entre las niñas con API no es superior a la de la población de su entorno.
Premature adrenarche is defined as the development of pubic and/or axillary hair and/or adult-like apocrine odour before age 8 years in girls and 9 years in boys. Idiopathic premature adrenarche (IPA) is diagnosed after ruling out other causes of excessive androgen production, such as adrenocortical or gonadal sex hormone secreting tumours, late-onset congenital adrenal hyperplasia or exogenous sources of androgens.1 Therefore, IPA is a diagnosis of exclusion.1,2
The exact prevalence of IPA in the general population is unknown,3 but it is a fairly frequent reason for medical visits, with a female-to-male ratio of approximately 9:1.4
To date, studies have analysed potential associations of IPA with a history of small for gestational age (SGA) or the presence of obesity and various metabolic disorders.1 A physiological hypothesis that attempts to explain the association between SGA and IPA proposes that malnutrition in the prenatal period may trigger a series of epigenetic changes that would alter the function of the adrenal gland after birth. To this, we need to add the potential impact of the rapidity of catch-up weight and height gain in the postnatal period.2,5 This hypothesis has been evaluated in studies conducted in cohorts of Catalonian girls with metabolic disorders, a history of SGA and androgen excess in childhood.6–9 Other studies have reported elevated serum levels of dehydroandrosterone sulfate (DHEA-S) during childhood and adolescence in girls with a history of SGA.1 However, there is no conclusive evidence that a history of SGA may directly predispose to the development of IPA. This may be due to the existing studies having been conducted in different populations, with different sample sizes and different criteria for the definition of SGA.2,10
The potential association between metabolic disorders, blood pressure and IPA has not been studied thoroughly. Idiopathic premature adrenarche results from an excess of adrenal androgen precursors, mainly DHEA-S. Some studies have found an association between the development of insulin resistance with elevated levels of DHEA-S.11 In fact, the presence of insulin resistance is a consistent finding in studies of girls with IPA, but there is some controversy in regards to the association of IPA with dyslipidaemia and high blood pressure (BP).2
There is also a degree of disagreement on the relationship that may exist in cases of obesity concurrent with IPA. For instance, on one hand, some studies have found an association between obesity and high serum or urine levels of DHEA-S. Furthermore, it seems that obesity and overweight are more prevalent in the IPA population compared with the general population. On the other hand, there are also studies that did not find evidence of these associations.1,2,10
Some authors believe that polycystic ovary syndrome (PCOS) may originate during pregnancy but manifest starting in adolescence.2,12 Consistent with this hypothesis, 45% of a cohort of Catalonian girls with IPA and a history of SGA progressed to PCOS at a later age.13
Considering the disparity in the evidence on the association between IPA with a history of SGA and metabolic disorders, we thought it would be relevant to analyse a sample of girls that met the diagnostic criteria for IPA. Thus, we hypothesised that girls with IPA would be more likely to have a history of SGA compared to the general population, and also be at higher risk of overweight or obesity and metabolic disorders. To evaluate this hypothesis, we set the following objectives: a) to analyse the association between IPA and a positive history of SGA; b) to assess the prevalence of overweight and obesity in girls with IPA; c) to determine the prevalence of high blood pressure and of changes in lipid and carbohydrate metabolism in girls with IPA.
Patients and methodsStudy designCross-sectional descriptive study.
SettingPaediatrics Department of the Hospital Universitario Fundación Jiménez Díaz.
Sample size calculationConsidering that the prevalence of SGA in the general population is estimated at 3%–5%,14 obtaining a difference in prevalence of at least 10% between the general population and girls with IPA required the inclusion of at least 53 girls with diagnostic criteria for IPA in the study cohort, for an α level of 0.05 and a power β of 80%.
Inclusion criteriaGirl without underlying disease and not undergoing chronic treatment that met the diagnostic criteria for IPA:
- -
Development of pubic and/or axillary hair and/or adult-like apocrine odour before age 8 years.
- -
Absence of the larche.
- -
Normal ACTH levels (to rule out the presence of late-onset congenital adrenal hyperplasia).
- -
Absence of ovarian or adrenal tumour (normal abdominal and pelvic ultrasound, androgen levels within normal ranges).
We reviewed the medical records of patients managed in the Department of Paediatrics of the Hospital Universitario Fundación Jiménez Díaz between January 2007 and May 2015 with a diagnosis of suspected IPA, without introducing, modifying or removing any data in the IT system and not entering into direct contact with patients to gather information. The project was approved by the Clinical Research Ethics Committee of the Hospital Universitario Fundación Jiménez Díaz.
Variables for which data were collected- -
Family history: height of both parents (cm), maternal age of menarche (years), family history of PCOS, family history of hyperandrogenism (considered positive if either a “history of hyperandrogenism” or a “history of female relatives with excessive body hair with a typically male distribution” was documented) and history of fertility problems in female relatives (considered positive if “sterility,” “infertility” or difficulties getting pregnant were documented).
- -
Personal history: ethnicity, country of birth, pregnancy, gestational age (weeks), type of delivery (spontaneous vaginal, caesarean or instrumental delivery), birth weight (in grams), birth length (in cm). We calculated the z-scores for the birth weight and length using reference tables for the Spanish population15 (Table 1).
Table 1.Perinatal characteristics of patients with IPA.
Variable Mean (95% CI) Gestational age 39.2 (38.86–39.53) Birth weight (g) 3100 (630)a Birth weight z-score 0.01 (−0.26 to 0.28) Birth length (cm) 49.74 (49.23–50.24) Birth length z-score 0.21 (−0.08 to 0.50) Prevalence Type of delivery Frequency Spontaneous vaginal 46/76 Caesarean 20/76 Instrumental 10/76 Small for gestational age 2/76 (2.72%) CI, confidence interval; IPA, idiopathic premature adrenarche.
- -
Demographic data, anthropometric characteristics and BP at diagnosis: chronological age (decimal age), age of onset of IPA (decimal age), height (cm), weight (kg), body mass index (BMI), BP (mm Hg), pubertal stage (based on the Tanner scale), bone age (assessed by the Greulichand Pyle method). We calculated the weight and height z-scores based on reference tables for the Spanish population.16 We also calculated the BP z-score using tables developed by an expert panel as reference.17
- -
Blood chemistry variables (after 12h of fasting at the time of diagnosis): glucose (mg/dL), fasting insulin (μIU/mL), HbA1C (%), total cholesterol (mg/dL), LDL cholesterol (mg/dL), HDL cholesterol (mg/dL), triglycerides (mg/dL), transaminases (IU/L), androstenedione (ng/ml), testosterone (ng/mL) and DHEA-S (μg/dl), baseline and 30- and 60-min peak 17-OH-progesterone levels after stimulation with 250μg ACTH (ng/mL).
- -
Criteria for SGA: birth length and/or weight at least 2 SDs below the mean (z<−2).15
- -
Criteria for overweight and obesity:
- •
Criteria set by the Spanish Ministry of Health in 2006 using BMI tables from the 1988 Spanish study16: a) normal weight: BMI between 3rd and 90th percentiles; b) overweight: BMI between 90th and 97th percentiles; c) obesity: BMI above the 97th percentile.
- •
Cole et al. criteria from 200018 and WHO criteria from 2007 modified according to BMI tables from a Spanish cross-sectional study published in 200819: a) normal weight: BMI between 3rd and 85thpercentiles; b) overweight: BMI between 85th and 95th percentiles; c) obesity: BMI above the 95th percentile.
- •
- -
Criteria for alteration of lipid metabolism: we calculated z-scores for total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides based on the reference tables.20 Thus, we defined hypertryglyceridaemia as trigyceride levels above the 95th percentile, and low HDL cholesterol as levels of it below the 5th percentile.
- -
Criteria for insulin resistance: we calculated the HOMA index using the formula [(glucose (mmol/L)×baseline insulin (μIU/L)]/22.5. We defined insulin resistance as a baseline insulin of less than 15μIU/mL and/or a HOMA index of less than 3.5.21
- -
Criteria for high blood pressure: we defined high BP as a systolic BP (SBP) and/or diastolic BP (DBP) above the 95th percentile for age, sex and height.17
We used SPSS version 19.0 to build a database with the data obtained for all the variables. We then performed a descriptive analysis of quantitative variables, calculating the mean and expressing the results with their 95% confidence intervals. Subsequently, we used the Kolmogorov–Smirnov test to verify that the variables were normally distributed. For variables that were not normally distributed but whose histogram had a shape that was very similar to a Gaussian bell, we decided to use the mean and confidence intervals obtained in the descriptive analysis. However, for variables that did not follow a normal distribution and whose histogram revealed a clearly asymmetrical distribution, we thought it would be more appropriate to use the median and interquartile range. For qualitative variables, we performed a descriptive analysis to calculate the prevalence.
We performed a bivariate analysis by means of the Student t test, dividing the sample into two groups for comparison: patients with normal weight, and patients with excess weight, with the latter including both overweight (BMI between the 90th and 97th percentiles) and obesity (BMI>97th percentile) based on Spanish reference tables.16 In addition, we compared the prevalence of overweight and obesity obtained in our study with the results reported in the most recent round of the ALADINO study, conducted in 2013.22
ResultsOf the total of 80 girls that sought care for premature adrenarche, 3 had results in the ACTH test that suggested the presence of congenital adrenal hyperplasia, and were therefore excluded from the study. Later on, this diagnosis was confirmed by genetic testing, which revealed changes in the 21-hydroxylase gene known to cause late-onset congenital adrenal hyperplasia. Another girl was also excluded because she had manifestations suggestive of premature adrenarche, but the data in her records was insufficient to establish a definitive diagnosis of IPA.
Thus, the final sample comprised a total of 76 girls. Of all of them, 74 had been born in Spain, 1 in Brazil and 1 in Bulgaria. As for ethnicity, 69 (90.7%) were Caucasian, while 5 were of Latin American, 1 of African and 1 of Indian descent. The median chronological age of the sample was 7.59 years (7.31–7.87).
The mean maternal height was 162.94cm (161.51–164.36) with a mean z-score of 0.29 (0.05–0.54); the mean paternal height was 175.85cm (173.99–177.70) with a mean z-score of 0.15 (−0.16 to 0.45); the mean predicted genetic height was 163.04cm (161.67–164.41) with a mean z-score of 0.28 (0.04–0.52).
As for the maternal age at menarche, the mean was 12.2 years (11.86–12.54). There was a family history of PCOS in 27.3% of the patients, of hyperandrogenism in 37.5%, and of fertility problems in 16.7%.
Only 2 girls had a history of SGA, which amounted to a prevalence of 2.7% (Table 1).
None of the girls met the criteria for high blood pressure. We found insulin resistance in 21.2%. Of the patients in who a lipid profile had been performed, 13.95% met the criteria for hypertryglyceridaemia and/or HDL cholesterol below the 5th percentile (Table 2).
Anthropometric and blood chemistry values at the time of diagnosis.
| Variable | Media (IC 95%) | Variable | Mean (95% CI) | ||
|---|---|---|---|---|---|
| Decimal age (year) | 7.59 (7.32–7.87) | Waist circumference (cm) | 77.8 (72.48–83.12) | ||
| Height (cm) | 131.83 (129.74–133.92) | Waist circumference z-score | 2.19 (1.46–2.92) | ||
| Height z-score | 1.65 (1.45–1.85) | SBP (mm Hg) | 100.35 (97.28–103.42) | ||
| Weight (kg) | 32.24 (30.43–34.05) | SBP z-score | 0.13 (−0.15 to 0.41) | ||
| Weight z-score | 1.51 (1.20–1.82) | DBP (mm Hg) | 59.21 (57.19–61.23) | ||
| BMI (kg/m2) | 18.33 (17.67–18.99) | DBP z-score | 0.08 (−0.11 to 0.27) | ||
| BMI z-score | 0.54 (0.29–0.78) | BA (decimal age) | 8.56 (8.21–8.92) | ||
| Alkaline phosphatase (IU/L) | 285 (243.54–326.46) | BA-CA | 0.96 (0.78–1.15) | ||
| Baseline 17-OH-progesterone (ng/mL) | 0.79 (0.67–0.91) | Androstenedione (ng/mL) | 0.53 (0.44)a | ||
| Peak 17-OH-progesterone (ng/mL) | 2.60 (2.52)a | DHEA-S (μg/dL) | 78.25 (66.81–89.69) | ||
| Testosterone (ng/mL) | 0.12 (0.10)a | Lipid metabolism | CT (mg/dL) | 164.14 (154.74–173.54) | |
| CH metabolism | Glucose (mg/dL) | 76.58 (74.89–78.27) | TC z-score | −0.48 (−0.78 to−0.18) | |
| Baseline insulin (μIU/mL) | 11.22 (9.52–12.92) | HDL (mg/dL) | 51.8 (48.37–55.23) | ||
| Glycated haemoglobin (%) | 5.22 (5.11–5.33) | HDL z-score | −0.65 (−0.92 to−0.39) | ||
| HOMA index | 2.13 (1.78–2.48) | LDL (mg/dL) | 99.87 (90.87–108.86) | ||
| IR | Frequency (%) | LDL z-score | −0.14 (−0.46 to −0.18) | ||
| 21.2 | TG (mg/dL) | 61.77 (55.37–68.16) | |||
| ALAT (GPT) (IU/L) | 21.53 (19.23–23.83) | TGz-score | 0.53 (0.3–0.75) | ||
| ASAT (GOT) (IU/L) | 29 (8)a | ||||
BA, bone age; BMI, body mass index; CA, chronological age; CH, carbohydrate; CI, confidence interval; DBP, diastolic blood pressure; IR, insulin resistance; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
We found statistically significant differences in triglycerides, triglyceride z-scores, HDL cholesterol, HDL cholesterol z-score, the HOMA index and insulin in the group of girls with excess weight (Table 3).
Comparative analysis of girls with IPA and normal weight versus girls with IPA with overweight or obesity.
| Independent variable | Girls with normal weight n=57 | Girls with overweight or obesity n=19 | Statistical significance |
|---|---|---|---|
| Birth weight z-score | −0.06 (−0.36 to0.24) | 0.22 (−0.43 to 0.86) | NS |
| Chronological age | 7.55 (7.21–7.89) | 7.74 (7.28–8.19) | NS |
| Bone age | 8.43 (7.98–8.87) | 8.97 (8.46–9.47) | NS |
| Bone age-chronological age | 0.88 (0.67–1.09) | 1.20 (0.8–1.6) | NS |
| Total cholesterol (mg/dL) | 167.42 (157.5–177.35) | 159.12 (139.46–178.78) | NS |
| Total cholesterol z-score | −0.44 (−0.76 to−0.12) | −0.55 (−1.16 to 0.07) | NS |
| Triglycerides (mg/dl) | 55.81 (49.60–62.01) | 70.88 (58.10–83.66) | P<.05 |
| Triglycerides z-score | 0.33 (0.10–0.56) | 0.82 (0.38–1.27) | P<.05 |
| LDL cholesterol (mg/dL) | 100.43 (91.62–109.24) | 99.06 (79.55–118.58) | NS |
| LDL z-score | −0.16 (−0.47 to 0.14) | −0.11 (−0.82 to 0.59) | NS |
| HDL cholesterol (mg/dL) | 55.35 (50.52–60.17) | 47 (42.79–51.20) | P<.05 |
| Z-score HDL z-score | −0.42 (−0.8 to −0.04) | −0.96 (−1.31 to−0.62) | P<.05 |
| Hb1Ac (%) | 5.17 (5.02–5.32) | 5.31 (5.16–5.46) | NS |
| HOMA index | 1.74 (1.39–2.09) | 2.93 (2.23–3.63) | P<.05 |
| Glucose (mg/dL) | 75.77 (73.66–77.88) | 78.33 (75.43–81.24) | NS |
| Insulin (μIU/mL) | 9.25 (7.57–10.93) | 15.28 (11.94–18.60) | P<.05 |
| SBP (mm Hg) | 100.85 (97.11–104.59) | 99 (93.28–104.72) | NS |
| SBP z-score | 0.21 (−0.13–0.55) | −0.09 (−0.61–0.43) | NS |
| DBP (mm Hg) | 59.09 (56.62–61.57) | 59.53 (55.83–63.23) | NS |
| DBP z-score | 0.09 (−0.13–0.33) | 0.03 (−0.31–0.37) | NS |
DBP, diastolic blood pressures; IPA, idiopathic premature adrenarche; NS, differences not statistically significant; SBP, systolic blood pressure.
Statistically significant variables with P<.05 are presented in italics.
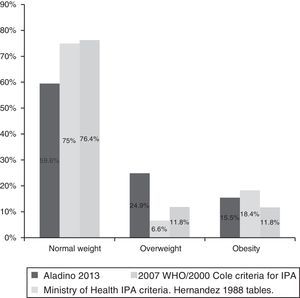
Applying the BMI categories established by the modified 2000 Cole/2007 WHO criteria, we found a prevalence of 11.8% for overweight and of 11.8% for obesity, that is, a prevalence of excess weight of 23.6%. When we applied the BMI categories of the Spanish Ministry of Health,16 we found a prevalence of overweight of 6.6% and a prevalence of obesity of 18.4% (excess weight, 25%) (Fig. 1).
DiscussionThe prevalence of SGA in the study sample did not exceed the prevalence reported for the general population, estimated at 3%–5%.14 When we reviewed the literature, we found inconsistent data regarding the association of IPA with SGA. Thus, a retrospective study that compared girls with premature pubarche (n=102) with girls who served as controls (n=83) found statistically significant differences in birth weight between the groups.23 The hypothesis that the authors proposed to explain this difference was that low birth weight could increase the risk of premature pubarche and other complications in the future. The same group of researchers designed a cohort composed of newborns meeting the criteria for SGA, defined as a birth weight z-score of less than −2, to analyse its causal relationship with the development of premature adrenarche and metabolic disorders.8 Along the same lines, an Australian study published in 200524 that defined SGA as a birth weight and/or a ponderal index below the 10th percentile based on reference charts25 found a history of SGA in 35% of girls with IPA. Conversely, 3 studies (one conducted in France, one in Scotland and one in Finland) did not find a greater prevalence of SGA in girls with IPA compared to controls.26–28 The discrepancies that exist between studies may be due to the application of different criteria to define IPA and SGA, as well as differences in sample size and the population under study.
In the study presented here, we analysed the prevalence of overweight and obesity applying different criteria: on one hand, a combination of the criteria set by the WHO in 2007 and the criteria proposed by Cole et al. in 2000,18 using as reference the BMI tables developed in a recent cross-sectional study conducted in Spain,19 with the purpose of having results based on international criteria; on the other hand, the criteria set by the Spanish Ministry of Health, which allowed us to compare our results to those of the most recent study of the prevalence of overweight and obesity in Spain: the ALADINO study.22 When we pooled the data for girls with overweight and girls with obesity, we found a prevalence of excess weight of 23.6% applying the modified 2007 WHO/2000 Cole criteria and of 25% applying the criteria of the Spanish Ministry of Health. The prevalence found in our study did not exceed the prevalence reported in a nationwide study as large as the ALADINO study, in which the combined prevalence of overweight and obesity reached 40.4%. In addition, the prevalence of obesity in our sample based on the criteria of the Ministry of Health (18.4%) was slightly greater than the prevalence of obesity in the ALADINO study (15.5%). The Australian study we cited above also assessed the prevalence of excess weight in girls with premature pubarche. The authors of that study used a BMI above the 85th percentile to define overweight and a BMI above the 97th percentile to define obesity based on their reference tables.24 Applying these criteria, they found overweight in 33.7% of the sample and obesity in 31.4%, that is, 65.1% of the girls had excess weight. These percentages were greater than those observed in the Australian general population. On the other hand, a study conducted in the Finnish population defined excess weight as a BMI above the 75th percentile. With this criterion, the authors found a significantly higher prevalence of excess weight in girls with IPA compared to controls.27 The heterogeneity of the criteria used to define IPA, overweight and obesity may influence the lack of consistency in the results reported in the literature.
At the time of the study, patients overall did not present with changes in BP, 14% had an abnormal lipid profile and 21.1% met the criteria for insulin resistance. Utriainen et al.27 analysed the prevalence of metabolic syndrome, assessed by means of the definition of the ATP III and the WHO 1998 criteria. They found that metabolic syndrome was more common in patients with IPA compared to controls, a difference that was statistically significant, and that the most significant factors associated with metabolic syndrome were excess weight and hyperinsulinism. Similarly, a study of Catalonian girls with a history of SGA, elevated levels of DHEA-S and low levels of SHBG (which, according to the authors’ hypothesis, are associated with IPA) found statistically significant differences in the ratio of the percentage of abdominal visceral fat to subcutaneous fat, hyperinsulinaemia and levels of IGF-1compared to the control group.9 Another study by the same research group8 found an association between a history of SGA and rapid weight gain between birth and age 2 years, which would predispose to and explain the greater percentage of visceral fat and prevalence of insulin resistance between ages 2 and 4 years. The main hypothesis of the authors is that the higher frequency of hyperinsulinaemia, hyperandrogenism and excess adiposity in girls with premature pubarche is associated with low birth weight.23 In our sample, the comparison of patients of IPA with normal weight and those with overweight and obesity showed significant differences in the levels of triglycerides, HDL cholesterol and baseline insulin and the HOMA index. This suggests that excess weight in girls with IPA is associated with changes in cardiovascular risk factors. However, determining whether these changes in cardiovascular risk factors are caused directly by IPA or result from excess weight would require further prospective studies with larger samples.
The main limitation of this study is the lack of a control group with which to make comparisons. Other limitations include not having performed an assessment of body composition and not having measured the levels of peptides associated with the pathophysiology of obesity.
We can conclude that in our study, girls with IPA are not more likely to have a history of SGA than the general population. On the other hand, the prevalence of overweight and obesity in girls with IPA is not greater than that in Spanish girls of similar age overall. Last of all, the changes we found in the lipid profile and carbohydrate metabolism of girls with IPA were significantly more prevalent in those that had overweight or obesity. In short, we think that it would be useful to contribute to the design of new multicentre studies with a larger number of patients from different regions in Spain and the establishment of homogeneous criteria for the definition of IPA, SGA and excess weight with the purpose of continuing to evaluate these potential associations.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Mejorado Molano FJ, Andrés Zallo L, Fornos Rodríguez M, Pérez Segura P, Gavela Pérez T, Sanz Calvo ML, et al. Estudio de la asociación de adrenarquia prematura idiopática con la presencia de alteraciones metabólicas y con antecedente de pequeño para edad gestacional. An Pediatr (Barc). 2017;87:253–259.