Proper nutrition is one of the primary objectives in the management of preterm infants. However, lack of evidence on the best strategy to achieve this objective has led to a great variability in feeding practices. This variability may be related to the differences in the incidence of complications, such as necrotising enterocolitis (NEC).
ObjectiveThe aim of this study is to assess the variability in clinical practice regarding enteral feeding in SEN-1500 Spanish network.
MethodAn observational study was conducted using a questionnaire sent out in 2013 requesting information about feeding very low birth weight (VLBW) neonates (bank milk, start time, trophic feeding, increases, fortifiers and probiotics).
ResultsResponses were received from 60 of the 98 hospitals. The response rate was higher in centres with more than 50VLBW/year (30/31). Just over two-thirds (67%) have feeding protocols, and 52% refer to variability within their unit. A milk bank is available in 25% of the units. First feeding occurs fairly evenly throughout first 48h, although it is delayed in lower gestational ages, even when there is no haemodynamic failure. In addition to haemodynamic instability there are other situations when the start is delayed (absence of breast milk, CIR, altered umbilical flow, asphyxia), while it is rarely delayed by absence of meconium or maintain an umbilical catheter. Half of those under 25 weeks begin directly with progressive increases instead of trophic feeding. Increases rarely reach 30mL/kg/day. Almost all use fortification and vitamins. There was a significant use of probiotics at the time of the survey.
ConclusionsThere is great variability in enteral nutrition policies in VLBW in Spain. Although some differences are justified by the lack of evidence, there are other interventions that have proven to be effective, such as evidence-based protocols or access to donor milk. Implementation in all the units could reduce the incidence of NEC and improve the nutritional status.
La nutrición adecuada es uno de los objetivos primordiales en el manejo de los recién nacidos prematuros. Sin embargo, la falta de evidencia en cuanto a cuál es la mejor estrategia para alcanzar este objetivo da lugar a que exista una gran variabilidad en las prácticas de alimentación. Esta variabilidad podría estar relacionada con las diferencias que existen en la incidencia de complicaciones como la enterocolitis necrosante (ECN).
ObjetivoValorar la variabilidad en las prácticas sobre alimentación entre las unidades neonatales de la red SEN-1500.
MétodoEstudio transversal, mediante cuestionario, solicitando información sobre alimentación del recién nacido de muy bajo peso (RNMBP) (leche donada, momento de inicio, trófica, incrementos, fortificantes, probióticos) en el año 2013.
ResultadosContestaron 60/98 hospitales; la tasa de respuesta fue mayor en centros con más de 50RNMBP/año (30/31). El 67% tienen protocolo de alimentación, el 52% refieren variabilidad en su unidad y el 25% disponen de leche donada. Se inicia la alimentación en las primeras 48h, aunque se retrasa en las edades más bajas aun en ausencia de fallo hemodinámico. Además de la inestabilidad hemodinámica hay otras situaciones por las que se demora su inicio (ausencia de leche materna, CIR, flujo umbilical alterado, asfixia), mientras que raramente se retrasa por ausencia de meconio o por mantener un catéter umbilical. Por debajo de 25semanas la mitad comienzan directamente con incrementos progresivos en lugar de nutrición trófica. Los incrementos raramente alcanzan 30ml/kg/día. Casi todos usan fortificantes y vitaminas. El uso de probióticos es excepcional.
ConclusionesExiste gran variabilidad en la política de alimentación del RNMBP entre las unidades neonatales españolas. Aunque algunas diferencias en las prácticas de alimentación están justificadas por la falta de evidencia, hay intervenciones que sí han demostrado su eficacia, como disponer de un protocolo de alimentación (basado en pruebas) o tener acceso a leche donada; su implementación en todos los centros podría disminuir la incidencia de ECN y mejorar el estado nutricional de los RNMBP.
Preterm newborns (NBs) are born during a critical period in growth and neurodevelopment. The goal of nutrition in very low birth weight (VLBW) newborns is to promote a growth that is similar to the one that would occur in utero without stressing their immature metabolism and excretory system. This is difficult to achieve in everyday practice due to the challenges posed by immature metabolic and digestive systems and the comorbidities present in these patients. As a result, many of them experience delays in extrauterine growth that compound the effects of previous intrauterine growth restriction. Suboptimal nutrition in this critical period may have irreparable consequences in both growth and neurodevelopmental outcomes.1,2
For this reason, the prevailing approach at present is the prevention of extrauterine growth retardation, to the extent possible, through the early and aggressive use of parenteral nutrition (with administration of nutrients that approximate those that would be received through the placenta) and initiation of enteral feeding at the earliest possible time.3
But the best way to implement this has yet to be determined. The evidence available on many of the procedures related to newborn nutrition is poor, which explains the broad variability observed in the approach to initiating and maintaining enteral feeding in different countries, different hospitals in a country and even different professionals in a hospital.4 This variability could be associated with differences in the incidence of postnatal malnutrition or necrotising enterocolitis (NEC). Several studies have shown that adequate standardisation of feeding protocols can reduce the incidence of NEC and/or improve nutritional outcomes.5,6
In Spain, theSEN-1500 network found evidence of postnatal growth retardation in VLBW newborns whose degree varied between neonatal units,1 as well as variations in the incidence of NEC, which was an exceptional occurrence in some facilities and more frequent in others (median, 6.9%; interquartile range, 0.00–8.3%).7
Few data are available on the feeding practices of Spanish hospitals. The aim of our study was to describe the variability of these practices in the different neonatal units that are part of the SEN-1500 network.
Materials and methodsIn 2013, we sent a questionnaire by electronic mail from the administrative office of the SEN-1500 network to the network's point of contact in each participating hospital, assigning each hospital a numerical code that was provided by an epidemiologist and that researchers were blinded to. It was possible for someone other than the point of contact to complete the questionnaire as long as the answers reflected the policies of the unit as opposed to the respondent's personal views. In order to improve the response rate, we mailed another general petition to all centres, as well as individualised requests for participation to the remaining hospitals.
We based our questionnaire in the items included in the study conducted by Klingenberg et al.,4 who surveyed preterm newborn feeding practices at the international level, and added a few items for variables that were not included in that study. The initial draft was reviewed by the members of the clinical management unit, and questions that were confusing were edited. The final questionnaire included 28 questions regarding the nutrition of newborns delivered at less than 32 weeks’ gestation and/or with birth weights of less than 1500g: 22 regarding feeding, 5 on parenteral nutrition, and 1 asking about changes in nutritional policies in the 2 previous years. Twenty-five of the items were multiple-choice single-answer questions, one was a multiple-choice multiple-answer question, and two were open-ended questions. Nine questions allowed the addition of comments. Five questions stratified the newborn population into three groups by gestational age (GA), adding approximate weights in parentheses: less than 25 weeks (<700g); 25–27 weeks (<1000g) and 28–31 weeks (<1500g).
In this article, we present our findings regarding feeding practices in year 2013 (timing of initiation, trophic nutrition [TN], feed increase volumes, donor milk, fortifiers and probiotics).
Since the SEN-1500 network includes hospitals offering different levels of care, we compared the practices between units that managed more than 50 NBs with birth weights of less than 1500g a year (“large” units) and the remaining units using the chi square test of independence, or its nonparametric equivalent when any of the frequencies was less than 5 (Fisher exact test). Every large unit and 24 out of the 29 “small” units managed newborns of less than 28 weeks’ GA (<1000g); we removed the remaining 5 from the analysis when we calculated proportions for those ages. We have presented the results as percentages.
Before we carried out the survey, we obtained the authorisation of the management of the SEN-1500 network. We present the pooled data, with no specific information for each facility, and in adherence with current laws and regulations on the protection of data (Law 41/2002 of November 14; Law 15/1999 of December 15). The study was approved by the Research Ethics Committee of Malaga.
ResultsThe survey was addressed to the 100 hospitals of the SEN-1500 network. Two were excluded because they did not have neonatal units at the time of the survey. Of the remaining 98, 60 responded (61% of surveyed hospitals), with a higher response rate in larger units (30/31; 97%) compared to the rest (29/67; 43%).
We obtained responses from hospitals in every autonomous community but Baleares, Extremadura and Cantabria; 95% were public hospitals and 73% were university hospitals.
Availability of a written protocol and variabilityOf all units, 67% (40/60) had a written protocol on newborn nutrition. Independent of the existence of such a protocol, 52% (31/60) reported interpersonal variability in the staff of their unit that was rated as very high in 3% (2/60), high in 5% (3/60), intermediate in 22% (13), low in 46% (28/60) and very low in 20% (12/60); 2 did not answer the question.
Availability of donor human milkOnly 8% of units (5/60) had a milk bank, and all 5 were large units; 17% (10/60) had access to donor milk from an external bank (5 large and 5 small units) and 75% (45/60) did not have access to donor milk.
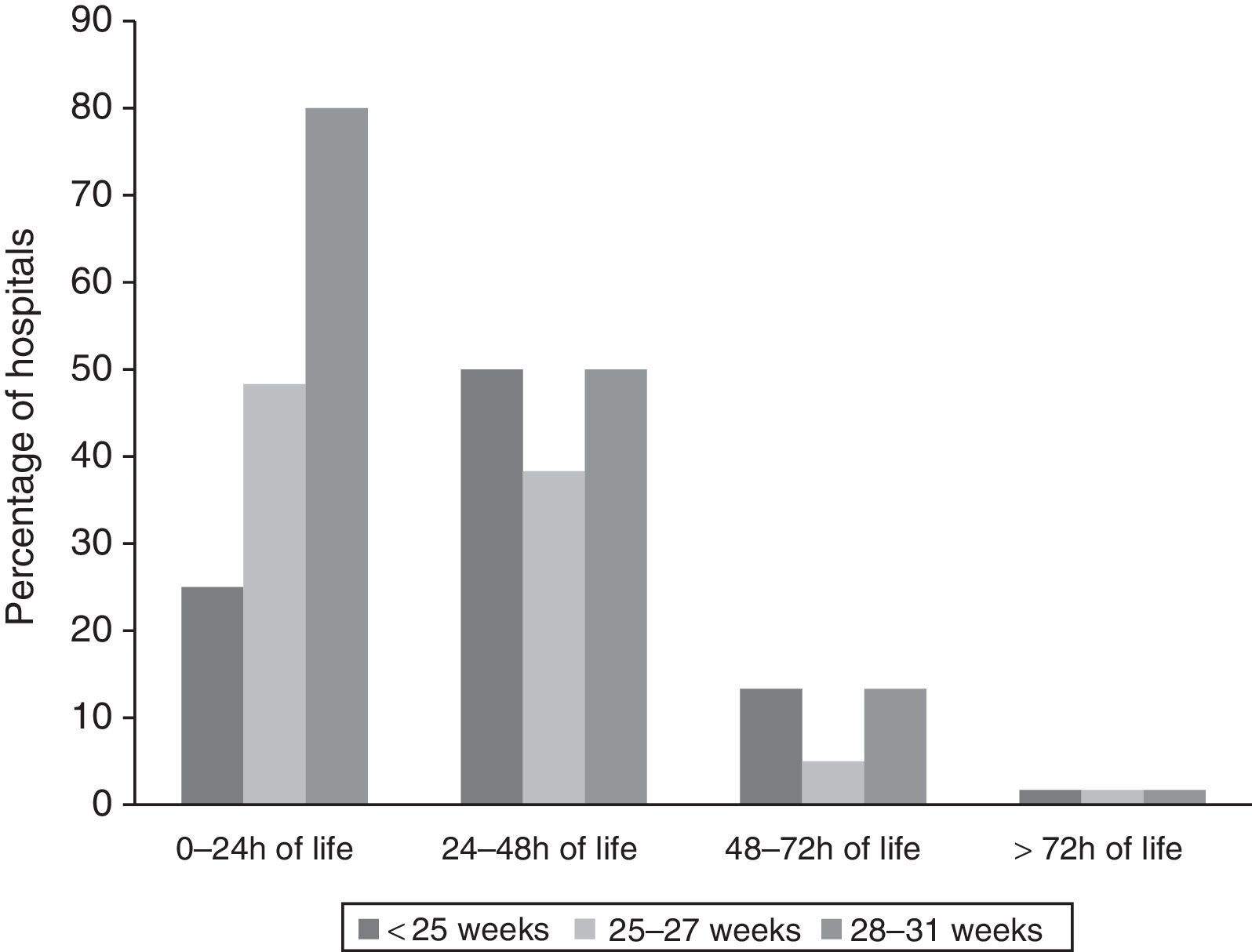
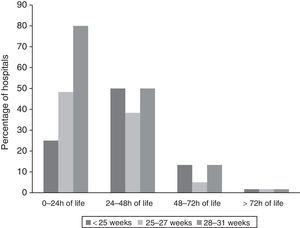
Timing of initiation of enteral feedingFig. 1 shows the timing of initiation of enteral feeding in haemodynamically stable VLBW newborns. Responses classified as “no answer” correspond to 5 hospitals that did not manage newborns of less than 28 weeks’ GA and 1 hospital that delayed initiation of feeding until human milk was available.
Trophic nutrition vs gradual feed advancement. Volume of increasesWe defined TN as enteral feeding not exceeding 20–24mL/kg/day for a period of 4–7 days. Some hospitals initiated enteral feeding with a period of TN followed by another period of gradual advancement, while others advanced feed volumes from initiation based on feeding tolerance without a preceding TN period.
The percentage of units that implemented TN decreased with increasing GA: 51% (29/55), 33% (19/57) and 5% (3/60) of units used TN in patients born at less than 25 weeks’, between 25 and 27 weeks, and between 28 and 31 weeks of gestation, respectively. Four hospitals applied TN periods of 2–3 days, below the minimum for our definition of TN.
All hospitals increased feed volumes by 10–20mL/kg/day, except 11 where increases could be as high as 30mL/kg/day, although only in larger preterm newborns; these 11 advanced enteral feeding without an initial period of TN.
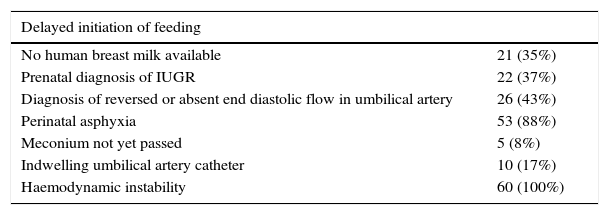
Situations in which units delayed the initiation of enteral feedingThere were marked differences in the significance assigned to various clinical indications for delaying or not delaying enteral feeding (Table 1). Many hospitals reported taking into account a combination of several of these factors in the decision-making process.
Situations in which units would delay the introduction of enteral feeds.
| Delayed initiation of feeding | |
|---|---|
| No human breast milk available | 21 (35%) |
| Prenatal diagnosis of IUGR | 22 (37%) |
| Diagnosis of reversed or absent end diastolic flow in umbilical artery | 26 (43%) |
| Perinatal asphyxia | 53 (88%) |
| Meconium not yet passed | 5 (8%) |
| Indwelling umbilical artery catheter | 10 (17%) |
| Haemodynamic instability | 60 (100%) |
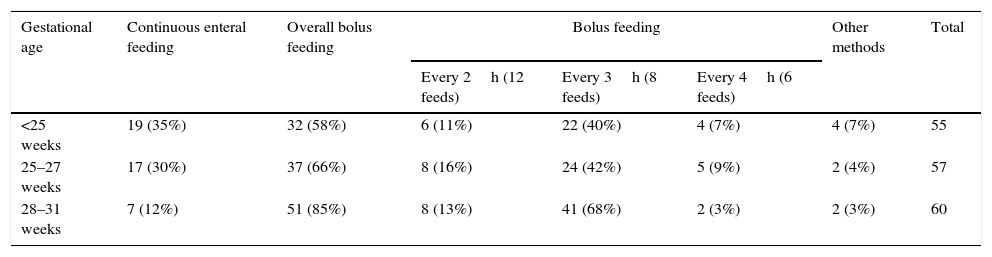
Table 2 shows the variations in the use of continuous vs bolus tube feeding, as well as in the volume and frequency of bolus feeds.
Mode of enteral feeding by gestational age.
| Gestational age | Continuous enteral feeding | Overall bolus feeding | Bolus feeding | Other methods | Total | ||
|---|---|---|---|---|---|---|---|
| Every 2h (12 feeds) | Every 3h (8 feeds) | Every 4h (6 feeds) | |||||
| <25 weeks | 19 (35%) | 32 (58%) | 6 (11%) | 22 (40%) | 4 (7%) | 4 (7%) | 55 |
| 25–27 weeks | 17 (30%) | 37 (66%) | 8 (16%) | 24 (42%) | 5 (9%) | 2 (4%) | 57 |
| 28–31 weeks | 7 (12%) | 51 (85%) | 8 (13%) | 41 (68%) | 2 (3%) | 2 (3%) | 60 |
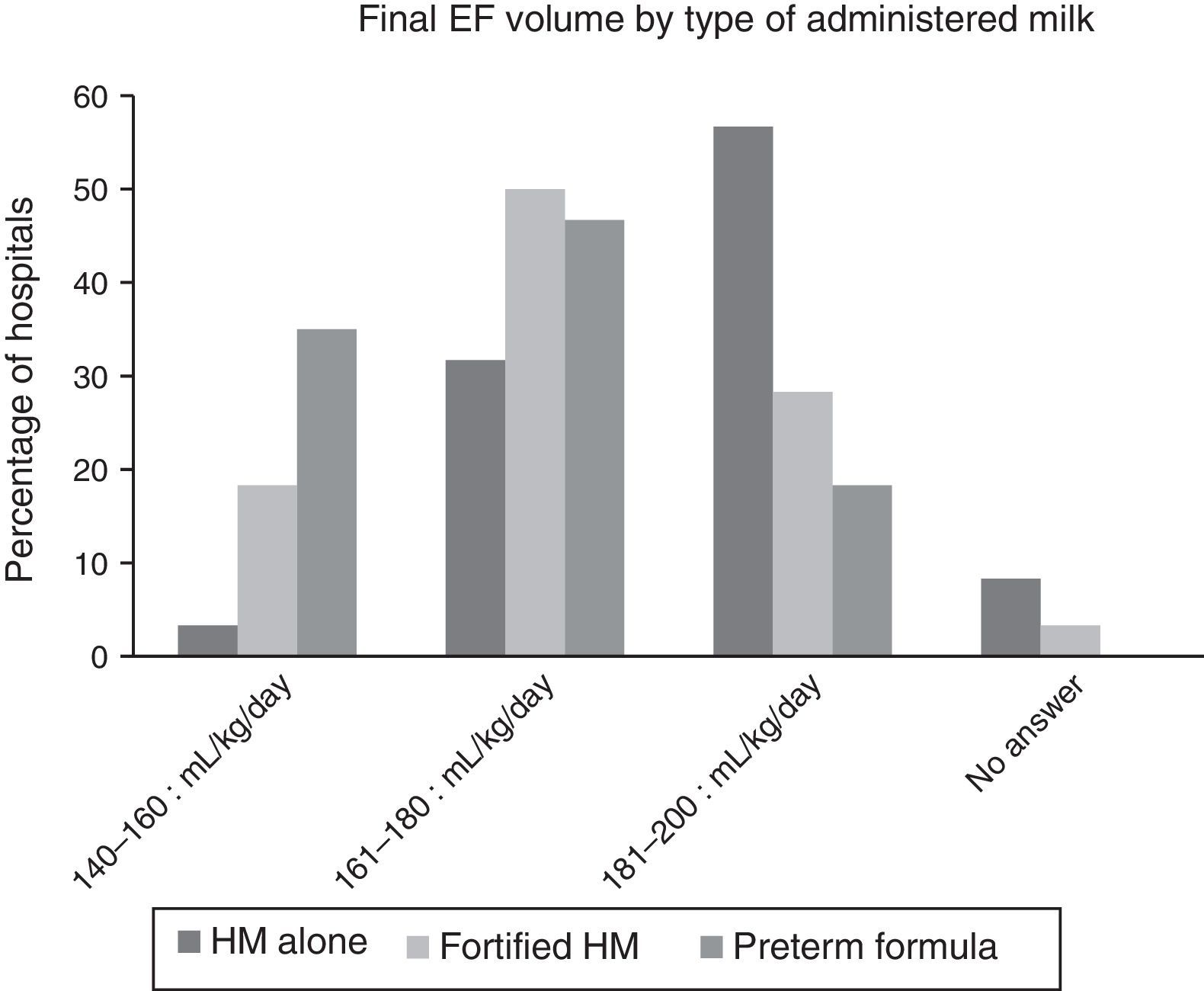
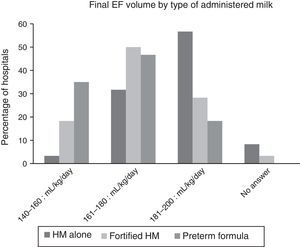
Fig. 2 shows the final volumes by type of nutrition. The volumes achieved with human milk alone were greater than those achieved with fortified human milk or with preterm formula, but there was still considerable variability within each of these types of milk.
FortifiersThe use of fortifiers was widespread, although the way in which they were used differed considerably between units.
Ninety-three percent of units (56/60) used fortifiers routinely and 3% (2/60) in selected patients, while 2 never used them. Of the 58 units that used fortifiers, 93% (54/58) used the administered milk volume as the criterion for initiating fortification (35/54 units initiated fortification when the feed volume reached 100–120mL/kg/day).
Twenty-four percent of the units (14/58) discontinued fortification when the patient reached a specific weight, 7% (4/58) at a specific age, 55% (32/58) when breastfeeding was established and 10% (6/58) at discharge, while 3% (2/58) maintained fortification post discharge.
Sixty-four percent (37/58) initiated fortification at low doses, increasing them progressively; the rest initiated fortification in full strength.
In 55% percent (32/58), fortifiers were added at the bedside prior to each feed; in 14% (8/58) milk was fortified in the unit and then kept refrigerated for 12–14h; in 31% (18/58), fortified milk was prepared in the milk-preparation/food services department—at the time of each feeding in half, and in advance and with refrigeration in the other half.
VitaminsNinety-five percent of the units (58/60) added vitamins to fortified human milk: 35% (21/60) a multivitamin, 38% (23/60) vitamin D and 20% (12/60) a combination of both.
Post-discharge feedingIn preterm newborns with birth weights of less than 1000g that maintained exclusive breastfeeding at discharge, 82% of units (49/60) did not recommend continued fortification of milk unless the patient's weight gain was inadequate, while 15% maintained fortification post discharge. In patients with mixed or artificial formula feeding at discharge, 72% of units (43/60) recommended the continued use of preterm formula; 15% (9/60) recommended the use of standard newborn formula, and 10% (6/60), standard formula for low birth weight infants. Two hospitals did not provide this information.
ProbioticsOnly 2 hospitals reported using probiotics at the time of the survey: 1 used them routinely, and 1 in specific cases. Both hospitals used a combination of Lactobacillus acidophilus and Lactobacillus biphidus.
Unit sizeAppendix A shows the percentages in the application of each of the measures described above by unit size (defined based on the admission of more or less than 50 VLBW newborns per year).
DiscussionThis study contributes evidence on enteral feeding clinical practices in newborns delivered before 32 weeks’ gestation and/or with birth weights of 1500g or less. Despite the intrinsic limitations of survey-based studies, we believe that it provides a fairly accurate reflection of current practices in Spain, and demonstrates the wide variability that exists in some of them.
The response rate was very high in large units, but lower in smaller units, probably because many of the latter do not manage or admit few newborns delivered before 28 weeks’ gestation and many questions in the survey specifically referred to this group. In those that responded, the feeding practices under study were similar to those reported by larger units.
There are some feeding practices that had not proven more effective than others, so that variability in their application is probably of little importance; however, there are measures for which there is sufficient evidence to make specific recommendations. Thus, when it comes to reducing the risk of NEC, the use of human milk, the implementation of a standard feeding protocol (both evidence-based recommendations) and probably the use of specific probiotics have a protective effect.
In 2013, only 25% of surveyed hospitals had access to donor milk. The use of formula doubles the risk of NEC compared to banked milk,8 so the increased availability of the latter could result in improved outcomes. A recent study highlighted the wide variability that exists in the use of maternal and banked donor milk for feeding VLBW newborns in Spanish hospitals, and in the measures that promote the availability of breast milk from the patient's mother.9
In neonatal care units, feeding regimens for VLBW preterm newborns should be standardised based on the most current evidence. This measure has been associated with a reduction in the incidence of NEC.5,10 In our survey, only 66% of the units had a written protocol and, even so, 37% of them acknowledged that there were variations in their feeding practices despite having one. This percentage rose to 75% in units without a written protocol.
The use of probiotics deserves specific attention. Recent studies have consistently found a reduction in the incidence of NEC and in mortality in association with certain strains and combinations of probiotics.11–16 At the time of our survey, only 2 units used probiotics, although we think it likely that this number has since grown. We should particularly emphasise the recommendation for their use in hospitals with a higher incidence of NEC.
The evidence is not as clear in regard to other practices, such as the timing of initiation, the indication of TN and its duration, or the volume of the feed increases during the advancement phase. While there are published clinical trials and systematic reviews on these aspects,17,18 the cases that we meet are often not comprehended in the inclusion criteria of these studies, which generally included few patients with extremely low birth weights or expressly excluded infants with growth restriction, Doppler abnormalities, haemodynamic problems or hypoxia. Some studies have found that feed volumes were advanced at a greater pace in patients that developed NEC.19 In addition, some observational studies found that the incidence of NEC improved with the use of protocols in which enteral feeding was introduced at a very slow pace,20,21 which could motivate us to extend this approach to newborns who are actually at low risk of NEC.
At times, the problem is that evidence from specific studies is applied to populations that were expressly excluded from them.22 Thus, it is worth noting that the timing of initiation of enteral feeding does not seem to have an impact on the risk of NEC and that TN is as safe as fasting, but only as long as the patient is haemodynamically stable; or that volume increases of 30mL/kg/day seem to be as safe as increases of 20mL/kg/day, although this has only been demonstrated in patients with birth weights of more than 1000g and following one week of TN.23,24 Perhaps a careful reading of recent clinical practice guidelines can help curb the erroneous application of scientific evidence and standardise some practices.23–30
In our study, we found a more cautious approach to the initiation of enteral feeding in the most preterm newborns, which was consistent with their increased risk of NEC. In most Spanish hospitals, enteral feeding is initiated early, in the first or second day of life. This approach is consistent with the current evidence, which recommends avoiding prolonged enteral fasting in haemodynamically stable VLBW newborns and initiation with trophic feeds to assist intestinal maturation.31,32
We did find differences in the subsequent advancement phase: even in the most immature NBs, half of the surveyed hospitals started increasing feed volumes from the first day, while the other half maintained TN for a period of time. The percentage of units in which volume increases were made from the beginning was higher in patients with greater gestational ages. The evidence on this subject is also poor: although some studies found that delaying feed advancement was not associated with an increased risk of NEC,18 others suggest the opposite.33
As for the volume of these increases, we found that few reached 30mL/kg/day, even in newborns with greater GAs. No unit advanced feeds at this high rate in patients born before 28 weeks’ gestation, which seems appropriate, as the current evidence is insufficient to support the recommendation of increases of 30 or more mL/kg/day in newborns with birth weights of less than 1000g.18
There is also considerable variability in the factors associated with delaying initiation of enteral nutrition. A large clinical trial demonstrated that in preterm newborns with gestations of less than 35 weeks, with a history of intrauterine growth restriction (IUGR) and abnormal umbilical artery Doppler waveforms and who do not require inotropic drug support, initiation of enteral feeding on day 2 post birth compared to day 6 was not associated with differences in risk of NEC and shortened the time to achievement of full enteral feeding.34 The lack of maternal or donor human milk can justify delaying enteral feeding in some facilities, but the presence of an indwelling umbilical artery catheter cannot.35
When it came to the manner in which milk was delivered, Spanish hospitals used both bolus and continuous nasogastric tube feeding. Although both methods may theoretically carry risks and benefits, the evidence is insufficient to recommend either method.36,37
Practices were more uniform when it came to the use of fortifiers and vitamins: most hospitals added them to human milk, a policy that is consistent with the current evidence, which shows that fortification during the hospital stay is associated with improved growth outcomes without increasing the risk of NEC.38 Fortifiers were introduced when feed volumes reached 100–120mL/kg/day, and discontinued once exclusive breastfeeding was achieved if the patient exhibited a favourable growth pattern. There was, however, more variability in how the milk was prepared: fortifiers undergo chemical changes over time after being added to milk, which warrants their addition at the time of the feeding. In 28% of units, previously prepared fortified milk was kept refrigerated for 12–24h.
In our analysis of the type of nutrition recommended at discharge, we found that in patients that were fed with artificial formula or a combination of formula and human milk, most hospitals maintained the use of preterm formula temporarily, while a lower percentage of facilities used standard or low-birth-weight newborn formulas. The evidence that is currently available is too inconsistent to support either approach. The volume intake of infants fed standard formula is probably greater, thus compensating the lesser concentration of nutrients. A recent meta-analysis suggested that nutrient-enriched formula may increase growth rates up to 18 months of age compared to standard term formula, but the evidence is insufficient to make a firm recommendation at this time.39
In the case of infants that were exclusively breastfed at the time of discharge, most hospitals did not recommend maintenance of fortification, and reserved the latter for infants with inadequate weight gain. At present, there is no evidence supporting routine fortification of milk following discharge.40
Limitations of the studyAs is the case with any survey-based study, the data we obtained may not accurately reflect actual practices. The sample under study only included hospitals that are part of the SEN-1500 network; however, we believe that the high response rate of the facilities that contribute the most patients to the network provides a good representation of this type of unit at the national level. On the other hand, given the lower response rate among smaller units or the possibility that the latter may have been underrepresented, it is possible that some practices may differ in hospitals offering lower levels of care. The data we collected corresponded to year 2013, and it is possible that some practices have since changed.
ConclusionsThere is a considerable variability in enteral feeding practices in newborns with birth weights of less than 1500g in Spanish neonatal units. When it comes to some practices, this variability is justified by a lack of evidence; however, the variability in other practices, such as the use of donor milk or the existence of evidence-based protocols, is not justified, as their use has been associated with a reduction in the incidence of NEC.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the collaboration of the following hospitals: Complejo Hospitalario Universitario de Albacete, Hospital General Universitario de Alicante, Hospital Torrecárdenas (Almería), Hospital de Cruces (Baracaldo), Hospital de la Santa Creu i Sant Pau (Barcelona), Hospital Universitario Valld’Hebron (Barcelona), Hospital Clínico (Barcelona), Instituto Universitario Dexeus (Barcelona), SCIAS Hospital de Barcelona (Barcelona), Hospital de Basurto (Bilbao), Hospital Infanta Margarita (Cabra), Hospital Universitario Puerta del Mar (Cádiz), Hospital Universitario Santa Lucía (Cartagena), Hospital General de Castellón, Complejo Hospitalario de Ciudad Real, Complejo Hospitalario Reina Sofía (Córdoba), Hospital General Universitario de Elche, Hospital Sant Joan de Déu (Esplugues de Llobregat), Hospital de Fuenlabrada, Hospital Universitario de Getafe, Hospital de Cabueñes (Gijón), Hospital Dr. Josep Trueta (Girona), Hospital Virgen de las Nieves (Granada), Hospital General de Granollers, Hospital Juan Ramón Jiménez (Huelva), Complejo Hospitalario Ciudad de Jaén, Hospital General de Jerez (Jerez de la Frontera), Hospital Juan Canalejo (La Coruña), Hospital Materno-Insular (Las Palmas), Hospital Severo Ochoa (Leganés), Hospital General de la Rioja (Logroño), Complejo Hospitalario de León, Hospital Universitario 12 de Octubre (Madrid), Hospital Universitario La Paz (Madrid), Hospital Universitario San Carlos (Madrid), Hospital Universitario Puerta del Hierro (Majadahonda), Complejo Hospitalario Carlos Haya (Málaga), Hospital Parque San Antonio (Málaga), Hospital Costa del Sol (Marbella), Hospital Universitario Virgen de la Arrixaca (Murcia), Hospital Central de Asturias (Oviedo), Hospital Virgen del Camino (Pamplona), Hospital del Bierzo (Ponferrada), Complejo Hospitalario de Pontevedra, Hospital Clínico Universitario de Salamanca, Hospital Donostia (San Sebastián), Complejo Hospitalario de Santiago (Santiago de Compostela), Hospital Virgen del Rocío (Seville), Hospital Nuestra Señora de Valme (Seville), Hospital Virgen Macarena (Seville), Hospital Universitario Juan XXIII (Tarragona), Hospital Universitario Nuestra Señora de Candelaria (Tenerife), Hospital Virgen de la Salud (Toledo), Hospital Clínico Universitario (Valencia), Hospital Universitario La Fe (Valencia), Hospital del Río Hortega (Valladolid), Complejo Hospitalario Xeral-Cies (Vigo), Hospital Txagorritxu (Vitoria), Hospital Clínico Universitario Lozano Blesa (Zaragoza), Hospital Miguel Servet (Zaragoza), Complejo asistencial de Zamora.
Please cite this article as: Moreno Algarra MC, Fernández Romero V, Sánchez Tamayo T, Espinosa Fernández MG, Salguero García E, Red SEN-1500. Variabilidad en las prácticas sobre alimentación enteral del prematuro entre hospitales españoles de la red SEN-1500. An Pediatr (Barc). 2017;87:245–252.