Although methylphenidate (MPH) used for treatment of attention deficit hyperactivity disorder (ADHD) are considered safe in healthy children and adolescents in the short and medium term, there is a widespread concern about long-term cardiovascular safety.
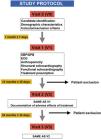
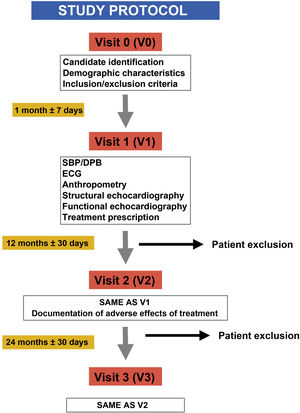
Material and methodsInterventional, prospective, longitudinal and comparative study with a crossover design to evaluate the cardiovascular impact of the treatment with MPH in healthy children and adolescents diagnosed with ADHD. A protocol for the cardiovascular evaluation was established at a basal point, after the first and the second year of the beginning with treatment based on the monitoring of blood pressure (BP) and echocardiographic follow-up of the systolic and diastolic functions, and structural cardiac properties.
Results73 patients completed the study, with an average age of 9 ± 2.6 years, 75.3% were male and the majority were thin (64.4%). We found an increase in Systolic and Diastolic BP of 3.7 ± 9 mmHg (P = 0.004) and 2 ± 11,5 mmHg respectively. There were no severe cardiovascular events. We didn’t find any echocardiographic alterations namely on the structural properties or parameters of systolic function. Regarding diastolic function, a significant increase in the isovo-lumic relaxation time (IVRT) (P = 0.046) and deceleration time (P = 0.016) was observed. However, no significant alterations in the parameters related to distensibility of the LV neither in the early diastolic pressure were found.
ConclusionFurther studies are needed to evaluate the impact of psychostimulants as a modifiable long-term Cardiovascular Risk Factor.
El metilfenidato (MTF) es un psicoestimulante que aumenta frecuencia cardiaca (FC) y presión arterial (PA), lo cual a largo plazo podría modificar la geometría del ventrículo izquierdo (VI) y alterar sus propiedades funcionales, principalmente la diastólica.
Material y métodosEstudio prospectivo, longitudinal y comparativo tipo caso-caso en niños y adolescentes sanos diagnosticados de trastorno por déficit de atención e hiperactividad (TDAH) tratados con MTF durante 3 años. Se valoró pre y postratamiento PAS/PAD, geometría ventricular, función sistólica y diastólica.
ResultadosIncluimos a 112 pacientes, completando 73. El 75,3% varones, entre 4–15 años (9 ± 2,6), con índice de masa corporal de 18,27 ± 3,75 y una dosis media de MTF de 0,9 ± 0,17 mg/kg/día. Objetivamos aumento de PAS/PAD de 3,7 ± 9 mmHg (p = 0,004) y 2 ± 11,5 mmHg, respectivamente. No tuvimos ningún evento cardiovascular grave, cambios estructurales ni variación en los parámetros de función sistólica estudiados. Sin embargo,encontramos un aumento discreto, pero progresivo y significativo del tiempo de relajación isovolumétrica del VI (p = 0,046) y del tiempo de desaceleración (p = 0,016) indicativos dealteración en la relajación. No observamos variación en los parámetros relacionados con la distensibilidad ni con las presiones diastólicas tempranas y ningún paciente cumplió criterios de disfunción diastólica.
ConclusionesEl incremento de PAS/PAD y las alteraciones de la relajación del VI objetivadas podrían ser un indicador precoz de una posible disfunción diastólica y riesgo cardiovascular a largo plazo.
Attention-deficit hyperactivity disorder (ADHD) is the most frequent neurodevelopmental disorder in the paediatric population, with a prevalence that ranges between 5% and 8% depending on the sociocultural characteristics of studied populations, the reviewed sources or the diagnostic criteria applied.1–3
In the past decade, there has been a significant increase in the prescription of psychostimulants in Western countries,4–6 and the number of potentially exposed patients is expected to increase on account of the expansion of their indications for other purposes7,8 and the increase in their illicit use, for instance, to improve cognitive performance in healthy individuals (“smart drugs”).9
Although clinical evidence supports the idea that the associated short- and medium-term cardiovascular risk in healthy children, adolescents and adults is low,10,11 following the publication in 2006 of a study that described a potential, albeit not proven, risk of severe cardiovascular events (cardiac arrest, sudden death, etc.),12 there has been widespread concern about their long-term cardiovascular safety.13–16
The literature describes a mean increase in heart rate (HR) of 5–10 beats per minute and in blood pressure (BP) of 4–5 mmHg,16,17 and a subset of children and adolescents (5%–15%) may experience larger increases or even have some form of cardiovascular complaint during treatment.17 Although there are authors that consider these cardiovascular effects irrelevant, it is important to consider that small increases in BP can gradually change the geometry of the left ventricle (LV) and alter its function. Diastolic dysfunction has been the most frequently reported type of cardiac damage in this context.18
Taking into account that we do not know whether the development of adverse cardiovascular effects depends on the dose or duration of exposure to psychostimulants and that methylphenidate (MTP) and lisdexamfetamine (LDX) sympathomimetic drugs whose effect on HR and BP is intrinsic to its pharmacological activity, we do not believe that the cardiovascular safety of these drugs has been well established.
Thus, we conducted a study with the following objectives:
- 1.
To assess whether the effects of psychostimulants on sympathetic tone and afterload have a medium-term impact on LV structure or function (systolic or diastolic).
- 2.
To assess the usefulness of serial ultrasound examinations for this purpose in children and adolescents with ADHD managed with psychostimulants.
- 3.
To determine the need of establishing a protocol for the medium-term cardiovascular follow-up of these patients.
We conducted a prospective, longitudinal, comparative and interventional case-crossover study to assess the cardiovascular impact of treatment with psychostimulants (MTP or LDX) at therapeutic doses commonly prescribed in clinical practice (0.8–1.5 mg/kg/day) in children and adolescents with a diagnosis of ADHD based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition text revision (DSM-IV-TR) and 5th edition (DSM-5) receiving pharmacotherapy for the first time (naïve patients) and managed at the paediatric neurology unit of a secondary level hospital in the southern Community of Madrid (Spain).
We enrolled patients from January 2011 through June 2014, and all patients completed the follow-up in July 2017.
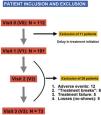
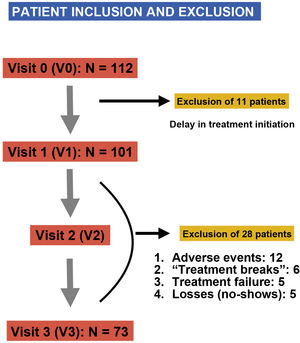
Exclusion criteriaKnown cardiovascular disease contraindicating the use of psychostimulants or that could affect study outcomes: congenital heart defect, ischaemic heart disease, sudden death, syncope, palpitations during rest, exercise intolerance, arrhythmia…
Cardiovascular risk factors that could affect diastolic function:
- 1.
Known high blood pressure (HBP)19: systolic blood pressure (SBP) or diastolic blood pressure (DBP) above the 95th percentile for age, sex and height based on the tables of the United States population published by the Task Force on BP20 and the European tables of Wühl and Stergiou21–23 for 21-h ambulatory BP monitoring and home BP monitoring, respectively.
- 2.
Medical comorbidities that affect diastolic function, such as type 1 diabetes24 and chronic kidney disease.25,26
- 3.
Obesity: defined as a body mass index (BMI) > 30.
- –
Discontinuation of treatment before the end of the study.
- –
Poor adherence to treatment or “treatment breaks”.
- –
Treatment with any other drug.
- –
Refusal of psychostimulant treatment or management with drugs other than stimulants, such as atomoxetine.
Blood pressure was measured at the clinic, always by the same provider, following the guidelines of the European Society of Hypertension and the European Society of Cardiology,27,28 using an oscillometric automated BP monitor and a sphygmomanometer cuff of a size appropriate for the width and length of the arm, taking 3 measurements throughout the visit and obtaining the definitive value by calculating the mean of the measures.
Sonographic evaluationThe evaluations were performed by the same paediatric cardiologist with the patient lying down on his left side and using the same equipment: the Esaote MyLab™ 25 Gold system (Esaote España S.A.) and a 3 or 5 MHz probe (multifrequency transducer) depending on the age of the patient and following the recommendations of the American Society of Echocardiography.29 The electrocardiogram cable was connected to the ultrasound system to define and measure the duration of events in the cardiac cycle.
- 1.
Structural echocardiography: morphology of cardiac chambers, valves and great vessels from different views.
- 2.
Left ventricular geometry: M-mode echocardiography, parasternal long-axis view, measurement of the posterior wall (PW) of the LV, the interventricular septum (IVS) and the left ventricular end-diastolic diameter (LVEDD). We calculated the left ventricular mass using the Devereux formula30 indexed to body surface area.
- 3.
Systolic function: we measured the LV ejection fraction (EF) and myocardial contraction fraction (MCF) and the tricuspid annular plane systolic excursion (TAPSE).
- 4.
Diastolic function: we obtained transmitral flow velocities in the apical 4-chamber view. We measured the early mitral inflow velocity (E), late mitral inflow velocity during atrial contraction (A) and the E/A ratio using pulse-wave Doppler, and calculated the E-wave deceleration time (DT) de using tissue Doppler imaging (TDI). Using TDI indices, we placed sample volumes on the lateral mitral and tricuspid annuli and the base of the IVS. We also measured the peak systolic, early diastolic and late diastolic velocities (E’ and A’, respectively) and calculated the E/E’ ratio. We measured the isovolumic relaxation time (IVRT) in the lateral walls of the LV. To reduce the impact of intraobserver variability, 3 measurements were taken for each parameter, and their mean was used as the final value in the analysis.
In this study, we defined LV diastolic dysfunction as an E/E’ ratio greater than 8 or an E/A ratio less than 1.0.
We considered IVRT values of 50 ± 9 ms and DT values of 142 ± 19 ms normal.29,31
Statistical analysisWe performed all the statistical analyses and generated all graphs with the software SPSS, version 23 (IBM SPSS Statistics). For all tests, we defined statistical significance as a P-value of less than 0.05.
We summarised qualitative data as absolute and relative frequencies and quantitative data as mean and standard deviation. We compared the parameters under study over time using repeated measures analysis of variance (ANOVA), introducing the different timepoints as a within-subjects factor. To compare the 3 timepoints, we used the Bonferroni correction for multiple comparisons.
To assess the effect of dosage on the observed changes in the parameters over time, we performed one-way repeated measures ANOVA introducing time as a within-subjects factor and the dose (a dichotomous variable with the categories dose > 0.9 mg/kg/day or dose < 0.9 mg/kg/day) as an intragroup factor.
We assessed changes in parameters between the baseline visit and visit 3 through the interaction of the time and dose factors (Fig. 1).
ResultsWe included a total of 112 patients, of who 39 (34.8%) dropped out of the study at different times and for different reasons (Fig. 2), so that a total of 73 patients completed the study. Participants were aged 4–15 years, with a mean age of 9 ± 2.6 years at the time of inclusion (visit 1 [V1]), 75.3% were male (n = 55) and most were underweight (64.4%), with a mean BMI of 18.27 ± 3.75. As regards the diagnosis of ADHD, the most frequent type was the inattentive type (56%), and 66% of participants had neuropsychiatric comorbidities, most frequently learning disorders (40%), followed by conduct disorders (18%), sleep disorders (4%) and mild cognitive impairment (4%). When it came to treatment, MTP was the most frequently used drug (92%), and osmotic-release MTP (OROS methylphenidate, Concerta, Janssen-Cilag, Spain) the dosage form prescribed most frequently (58%). We did not find differences in dosage between the two stimulants (MTP and LDX), with the prescribed dose ranging from 0.5 to 1.36 mg/kg/day and a mean dose of 0.9 ± 0.17 mg/kg/day. Table 1 summarises the demographic characteristics of the sample.
Demographic characteristics of participants.
| Included patients | 112 |
| Lost to follow-up (%) | 34.8% |
| Final sample | 73 |
| Age in years (mean ± SD) | 9 ± 2.6 |
| Male (%) | 75.3% |
| BMI (mean ± SD) | 18.27 ± 3.75 |
| ADHD type (%) | Inattentive, 56% |
| Combined, 43% | |
| Hyperactive, 2% | |
| Neuropsychiatric comorbidities (%) | 66% |
| Learning, 40% | |
| Conduct, 18% | |
| Sleep, 4% | |
| Mild cognitive impairment, 4% | |
| Psychostimulant drug (%) | Methylphenidate, 92% |
| Dose (mg/kg/day), mean ± SD | 0.9 ± 0.17 |
| Sociocultural level (%) | Low, 20.6% |
| Middle, 40.3% | |
| High, 39.1% |
ADHD, attention-deficit hyperactivity disorder; BMI, body mass index; MTP, methylphenidate.
When it came to the cardiovascular status at the beginning of the study, all patients had SBP and DBP values in the normal range for their sex, age and anthropometric measurements, with mean values of 104 ± 8.3 mmHg and 62.7 ± 8.3 mmHg, respectively. We did not find abnormalities in any of the parameters used to describe LV geometry (LVEDD, posterior wall, IVS, LV mass), and the ventricular mass calculated with the Devereux formula indexed to body surface area was normal. We also found no abnormalities in the parameters used to assess systolic function in the left or right ventricles (mean values: EF, 67.7 ± 5.5; MCF, 37.2 ± 4.5; TAPSE, 22.64 ± 2.48) and diastolic function was normal (mean E/E’, 5.5; mean E/A, 1.85) (Table 2).
Cardiovascular status at the beginning of the study.
| Mean | Range | SD | |
|---|---|---|---|
| LVEDD (mm) | 4 | 2–5.1 | 0.56 |
| PW (mm) | 0.537 | 0.38–1 | 0.112 |
| IVS (mm) | 0.563 | 0.33–1 | 0.128 |
| LVM (g) | 59.9 | 20.7–125 | 25.3 |
| EF (%) | 67.7 | 57–81 | 5.5 |
| MCF (%) | 37.2 | 29–49 | 4.5 |
| TAPSE (mm) | 22.6 | 17–34 | 2.5 |
| E (m/s) | 1.05 | 0.65–1.43 | 0.15 |
| A (m/s) | 0.57 | 0.4–0.8 | 0.12 |
| E/A | 1.85 | 1–3 | 0.42 |
| DT (ms) | 110 | 51–192 | 37 |
| IVRT (ms) | 57.3 | 32–108 | 13.2 |
| E/E’ | 5.5 | 3–9 | 1.31 |
A (wave), flow velocity during active atrial contraction; DT, deceleration time; E (wave): early diastolic inflow velocity; E/A, E/A ratio; E/E’, E/E’ ratio; EF, ejection fraction; IVRT, isovolumic relaxation time (left ventricle); IVS, interventricular septum; LVEDD, left ventricular end-diastolic diameter; LVM, left ventricular mass; MCF, myocardial contraction fraction; PW, posterior wall; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion.
We found a significant increase between V1 and visit 3 (V3) in SBP of 3.7 ± 9 mmHg (P = 0.004) and in DBP of 2 ± 11.5 mmHg (not statistically significant) independent of the dose (high: >0.9 mg/kg/day; low: <0.9 mg/kg/day) and the prescribed stimulant (MTP vs LDX).
Structurally, ventricular geometry and mass remained stable through the end of the study. The systolic function parameters in both ventricles (EF, 70.3 ± 5.1; MCF, 39.9 ± 4.6; TAPSE, 22.2 ± 2.95) did not experience significant changes at any of the time points. When it came to diastolic function, we found a small but progressive and significant increase in IVRT from 57.3 ± 13.2 in V1 to 62.4 ± 14.6 in V3 (P = 0.046) and in the percentage of patients with a prolonged IVRT (31%) by the end of the study period. However, we did not find significant changes in the parameters used to assess LV distensibility or in early diastolic pressures, and none of the patients met the definition criteria for diastolic dysfunction. Although we observed significant changes in the DT (P = 0.016), they were not clinically relevant, the E wave remained above the A with an E/A ratio above 1 throughout the follow-up, and there were also no significant changes in the E/E’ ratio (Table 3).
Changes in cardiovascular status through the study period.
| V1 | V2 | V3 | P (V1–V3) | |
|---|---|---|---|---|
| LVEDD (mm) | 4 ± 0.56 | 4.3 ± 0.56 | 4.7 ± 0.58 | 0.246 |
| PW (mm) | 0.537 ± 0.112 | 0.54 ± 0.09 | 0.545 ± 0.1 | 1.00 |
| IVS (mm) | 0.563 ± 0.128 | 0.561 ± 0.1 | 5.5 ± 0.1 | 1.00 |
| LVM (g) | 59.9 ± 25.3 | 61 ± 26.4 | 61.96 ± 25.7 | 0.61 |
| TAPSE (mm) | 22.6 ± 2.48 | 22 ± 3.4 | 22.2 ± 2.95 | 0.68 |
| EF (%) | 67.7 ± 5.5 | 68.9 ± 4.6 | 70.3 ± 5.1 | 0.02 |
| MCF (%) | 37.2 ± 4.5 | 38.9 ± 4.5 | 39.9 ± 4.6 | 0.01 |
| E (m/s) | 1.05 ± 0.15 | 1.01 ± 0.16 | 1.03 ± 0.16 | 1.00 |
| A (m/s) | 0.57 ± 0.12 | 0.57 ± 0.12 | 0.59 ± 0.13 | 0.95 |
| E/A | 1.85 ± 0.42 | 1.78 ± 0.52 | 1.74 ± 0.38 | 0.167 |
| DT (ms) | 110 ± 37 | 99 ± 24.9 | 96.3 ± 25.7 | 0.016 |
| IVRT (ms) | 57.3 ± 13.2 | 58,.99 ± 17.1 | 62.4 ± 14.6 | 0.046 |
| E/E’ | 5.5 ± 1.31 | 5.4 ± 1.16 | 5.23 ± 1 | 0.276 |
A (wave), flow velocity during active atrial contraction; DT, deceleration time; E (wave): early diastolic inflow velocity; E/A, E/A ratio; E/E’, E/E’ ratio; EF, ejection fraction; IVRT, isovolumic relaxation time (left ventricle); IVS, interventricular septum; LVEDD, left ventricular end-diastolic diameter; LVM, left ventricular mass; MCF, myocardial contraction fraction; PW, posterior wall; TAPSE, tricuspid annular plane systolic excursion.
Statistically significant results are presented in boldface (P < 0.05).
Data for visits 1, 2 and 3: mean ± standard deviation.
Methylphenidate is a psychostimulant that has been available in Europe since the 1950s, and while its prescription in Western countries has increased exponentially in the last decade,4–6 its long-term adverse effects have yet to be been fully established.
In January 2009, the Committee for Medicinal Products for Human Use of the European Commission published a review32 followed by recommendations to make prescribing for medicines containing MTP consistent and maximize their safety across the European Union, remarking on the need for additional studies analysing, among other aspects, their long-term cardiovascular impact in adults currently or formerly treated with MTP.32
Studies on the abuse of stimulants such as methamphetamine suggest potential cardiotoxicity associated with the imbalance caused by the increase in oxygen requirements that results from an increased HR and BP and the low availability of oxygen in heart tissue33,34 resulting from the ensuing vasoconstriction. In addition, excessive elevation of catecholamine levels can cause cardiac muscle necrosis, fibrosis or hypertrophy.34,35
Although there is no evidence that stimulants prescribed to treat ADHD increase cardiovascular risk in healthy young individuals,36 given that the effects of MTP and LDX are similar to those of illicit stimulants on account of their mechanism of action,37 it would seem reasonable to conduct studies to assess potential cardiovascular complications in the long term.
Compared to previous studies,12–17 ours is the first to make a prospective analysis of potential early changes in cardiac structure or function in the medium term (36 months) by means of serial echocardiography in children and adolescents with ADHD starting treatment with psychostimulants.
We designed a study protocol for follow-up and echocardiographic assessment that was similar to those of previous studies on various chronic diseases in paediatric patients,19,24–26 although we set very stringent exclusion criteria to minimise the effect of potential confounders such as obesity, pre-existing HBP, heart disease etc.
We obtained a fairly large sample,24–26 although it had certain epidemiological peculiarities,1–5 such as the predominance of the inattentive type of ADHD (56%) over the combined type and the higher prevalence of learning disorders compared to conduct disorders (40% vs 18%) as the most frequent comorbidity. A possible explanation is that patients with combined ADHD would have seem to need more urgent treatment due to the predominance of externalizing behaviours in this type of ADHD and that 11 patients included initially (V0) refused to attend visit 1 and were excluded from the study. Although the sample was homogeneous as regards the socioeconomic and cultural level of participants, the symptoms and the impairment caused by ADHD, these epidemiological characteristics may not be representative of the patients seen in everyday clinical practice, which may preclude the extrapolation of cardiovascular safety outcomes in the sample to the total population.
Another important epidemiological aspect that needs to be considered due to its potential impact on long-term cardiovascular safety is that, from a somatometric standpoint, most patients (64.4%) were underweight based on their BMI (<18.49) and, as described in other nationwide studies,38 there was an overall decrease in BMI of 0.5 ± 2.2 points that was mainly due to weight loss.
This is important because excess weight is associated with an increased risk of cardiovascular disease independently of other cardiovascular risk factors (CVRFs), in men but especially in women. It also promotes the development of other CVRSs such as HBP, diabetes or dyslipidaemia (especially hypertriglyceridemia, low HDL levels and increased LDL levels) and ventricular hypertrophy, and increases systemic inflammation.39,40 Given that obesity in childhood or adolescence is likely to persist in adulthood, the fact that none of the patients in our sample had obesity or any of the associated CVRFs may reinforce our findings.
As concerns cardiovascular outcomes, although we found a progressive increase in SBP and DBP that was expected16,17 and measurements were made in adherence with current recommendations,22,27,28 we believe that our findings should be interpreted with caution.
Compared to previous evidence, and consistent with a yet-unproven hypothesis,41 the increase in SBP and DBP was independent of the type of stimulant used and its dose (high vs low).
Since there were no serious cardiovascular events and the echocardiographic evaluation did not find structural or functional abnormalities that would meet the definition of systolic or diastolic dysfunction in the 3-year follow-up, it would be reasonable to conclude that both psychostimulants are safe from a cardiovascular standpoint.
However, since the BP changes caused by psychostimulants do not immediately translate to clinically significant effects, we believe that the data we contribute on the early stages of myocardial relaxation abnormalities are relevant and warrant further research in larger, randomly selected samples with control groups and longer follow-up to confirm these results.
From a pathophysiological standpoint, our findings suggest that increases in BP, even if not immediately clinically significant, may lead to myocardial remodelling that is not detectable by echocardiography but can be discerned in Doppler imaging.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García Ron A, Rodriguez Mesa M, Arias Vivas E, Bote Gascon M. Impacto del tratamiento con metilfenidato sobre las propiedades funcionales y estructurales del ventrículo izquierdo a medio plazo en el trastorno por déficit de atención e hiperactividad. An Pediatr (Barc). 2022;96:43–50.
Previous presentations: This study was presented and received an award as one of the best oral presentations at the 66th Congress of the Asociación Española de Pediatría, June 7–9, 2018, Zaragoza, Spain.