To investigate the blood lead levels (BLLs) and faecal lead levels (FLLs) in children with various functional gastrointestinal disorders (FGIDs) and compare them with controls.
Patients and methodsOne hundred and two children with FGIDs defined by the Rome IV criteria, aged 4–18 years, and one hundred and two sex matched healthy children were enrolled in the study. Children with FGIDs were divided into three subgroups as functional constipation (FC) (n = 36), functional abdominal pain (FAP) (n = 36) and functional nausea (FN) (n = 30). The lead levels were measured using atomic absorption spectrometer.
ResultsThe median BLLs in the FGIDs group was significantly higher than in controls (5.12 and 1.77 µg/dL, respectively). The BLLs were above 5 µg/dL in 51,9% of children with FGIDs. There was statistically significant difference in BLLs between FC subgroup and the other subgroups (FAP and FN) (p = 0.003, p < 0.001 respectively). The FLLs in the FGIDs group was significantly higher than in controls (28.08 and 0.01 µg/g, respectively). There was no significant difference in FLLs between FC subgroup and the other subgroups (p = 0.992, p = 0.989 respectively). No significant relation found between BLLs and FLLs of the FGIDs group (p = 0.123).
ConclusionThis study revealed that children with FGIDs had higher BLLs and FLLs than controls and also more than half of children with FGIDs had BLLs ≥5 µg/dL which is considered as toxic level. These results might revive the question of whether or not clinicians need to evaluate routine BLLs in children with FGIDs.
El objetivo del estudio fue determinar los niveles séricos y fecales de plomo en niños con distintos trastornos digestivos funcionales (TDF) en comparación con controles sanos.
Pacientes y métodosLa muestra incluyó a 102 niños de 4–18 años con TDF definidos mediante los criterios de Roma IV y a 102 controles sanos emparejados por edad y sexo. Los niños con TDF se dividieron en 3 subgrupos: estreñimiento funcional (EF) (n = 36), dolor abdominal funcional (DAF) (n = 36) y náuseas funcionales (NF) (n = 30). Los niveles de plomo se midieron mediante espectrometría de absorción atómica.
ResultadosEl nivel de plomo en sangre (NPS) mediano fue significativamente mayor en niños con TDF en comparación con controles (5,12 vs. 1,77 µg/dl). Los NPS superaron los 5 µg/dl en el 51,9% del grupo TDF. Se observó una diferencia estadísticamente significativa en los NPS entre el subgrupo con EF y los otros dos subgrupos (DAF y NF) (p = 0,003 and p < 0,001, respectivamente). Los niveles de plomo en heces (NPH) fueron significativamente mayores en niños con TDF en comparación con controles (28,08 vs. 0,01 µg/g). No hubo diferencias significativas en los NPH entre el subgrupo de EF y los otros subgrupos (p = 0,992 y p = 0,989). No se encontró una correlación significativa entre los NPS y los NPH en niños con TDF (p = 0,123).
ConclusiónEl presente estudio demostró que los niveles séricos y fecales de plomo eran superiores en niños con TDF en comparación con controles, y que más de la mitad de los niños con TDF tenían NPS ≥ 5 µg/dl, que se consideran tóxicos. A la vista de estos resultados, cabe replantearse si los clínicos han de determinar los NPS de manera rutinaria en niños con TDF.
Functional gastrointestinal disorders (FGIDs) are a heterogeneous group of diseases characterized by persistent and recurring gastrointestinal (GI) symptoms with no identifiable organic cause.1 Due to the absence of identifiable markers, the Rome Foundation developed symptom-based criteria for diagnosis of FGIDs. The Rome criteria for children with FGIDs were first proposed in the mid-1990s and last updated in 2016 (Rome IV).2 Recently, the prevalence of FGIDs based on Rome IV criteria has been reported as 21%.3 Although more than 20 FGIDs have been identified, the Rome IV Classification is divided into 8 categories, which are oesophageal disorders, gastroduodenal disorders, bowel disorders, centrally mediated disorders of gastrointestinal pain, gallbladder and sphincter of Oddi disorders, anorectal disorders, childhood FGIDs: neonate/toddler, and childhood FGIDs: child/adolescent.4 Within these categories, the most frequent FGID subcategory found in the paediatric age group is functional constipation (FC).5 The diagnosis of FGIDs requires a thorough history-taking and physical examination and detection of warning signs through the application of the Rome criteria. Nevertheless, the use of standard diagnostic tests may be necessary to rule out organic illnesses or to investigate possible structural problems.6
The amount of lead in the human body has increased along with industrialization. Lead can be found throughout the environment in contaminated soil, air or water. Once lead gets into the body through breathing or swallowing, it promptly circulates in the blood and is distributed to all tissues.7 As the absorption of lead from the gastrointestinal tract varies depending on nutritional status, age and the form and particle size of lead, infants, undernourished children with calcium and iron deficiency, pregnant women and individuals subject to high occupational exposure are at higher risk of experiencing the toxic effects of lead.8 Although lead toxicity in both children and adults has an adverse effect on many organ systems, the signs and symptoms of lead exposure mainly involve the nervous system/cognition and the digestive system.9 The gastrointestinal (GI) manifestations of lead poisoning include chronic or recurrent abdominal pain, nausea, vomiting, constipation, bloating, anorexia and weight loss.10,11 Since lead is excreted from the body primarily through the urine, faeces, saliva, sweat, hair and nails, lead poisoning can be easily diagnosed with blood tests.12 While the Centers for Disease Control and Prevention (CDC) have established the blood lead level (BLL) threshold for children at 5 μg/dL, it has yet to establish reference values for faecal or urine lead levels.13
Few studies have measured BLLs in children with gastrointestinal complaints. Most of them focused on BLLs in children with constipation and abdominal pain that were not categorised using the Rome criteria.14,15 In addition, no studies have assessed both blood and faecal lead in children with FGIDs. Therefore, the aim of our study was to measure blood as well as faecal lead levels in children with various FGIDs such as FC, functional abdominal pain (FAP) and functional nausea (FN). To our knowledge, this is the first report on BLLs and faecal lead levels (FLLs) in children with different subtypes of FGIDs.
Sample and methodsWe conducted a cross-sectional study at the Department of Paediatric Gastroenterology of the Karabuk Training and Education Hospital in Turkey between January and September of 2018. The study included 102 children aged 4–18 years with FGIDs defined by the Rome criteria. The control group consisted of 102 healthy children matched for age and sex managed at the Healthy Child Clinic. We ruled out the presence of organic and metabolic diseases in all study participants through a thorough history-taking and physical examination and laboratory tests including a complete blood count, blood chemistry tests, erythrocyte sedimentation rate (ESR) and urine and stool tests.
We categorised children with FGIDs into 3 subgroups. The first subgroup consisted of 36 children with FC. Children received a diagnosis of FC based on the Rome IV criteria if they had 2 or more of the following symptoms occurring at least once per week for a minimum of 1 month and with insufficient criteria for diagnosis of irritable bowel syndrome: 1) 2 or fewer defecations in the toilet per week in a child of a developmental age of at least 4 years. 2) At least 1 episode of faecal incontinence per week. 3) History of retentive posturing or excessive volitional stool retention. 4) History of painful or hard bowel movements. 5) Presence of a large faecal mass in the rectum. 6) History of large diameter stools that can obstruct the toilet. The second subgroup consisted of 36 children with FAP. Children received a diagnosis of FAP based on the Rome IV criteria if they experienced all of the following at least 4 times per month: 1) Episodic or continuous abdominal pain that does not occur solely during physiologic events (such as eating, menses). 2) Insufficient criteria for irritable bowel syndrome, functional dyspepsia, or abdominal migraine. The last subgroup consisted of 30 children with FN. Children received a diagnosis of FN based on the Rome IV criteria if they met all of the following: 1) Bothersome nausea as the predominant symptom, occurring at least twice per week, and generally not related to meals. 2) Not consistently associated with vomiting. For the assessment of BLLs and FLLs in children in prepubertal and postpubertal ages, we divided the FGID and control groups into 2 age subgroups: 4–11 years and 12–18years.
The study was approved by the Ethics Committee for Non-invasive Clinical Research of Karabuk University, under file no. 2018/1.4. All participants provided written informed consent.
Measurement of blood lead levelsWe collected fasting venous blood samples (3 mL) into plastic tubes containing EDTA as an anticoagulant and stored them at −20 °C until they were analysed. For the analysis, samples were thawed, followed by addition of 7 mL of 65% nitric acid (Merc 100456) and 2 mL of 30% hydrogen peroxide (Merc 108600), then vortexed and digested in a microwave oven (CEM MARS 5X press) at 160 degrees for 30 min. The digested contents were passed through a 0.45 μm injector tip filter (Whatman Syringe Filters, 0.45 μm). After filtration, each sample was transferred to a 15 mL Falcon tube. Serum lead levels were measured using a graphite furnace atomic absorption spectrometer (THERMO SCIENTIFIC ICE 3400 AA), with a limit of detection for lead in blood of 0.1 µg/L.
Measurement of faecal lead levelsSamples of 30 mL of first-morning stool were collected in plastic bottles, washed in 2% nitric acid and stored at −70 °C until they were analysed. For the analysis, samples were thawed, 7 mL of 65% nitric acid (Merc 100456) and 2 mL of 30% hydrogen peroxide (Merc 108600), then digested in a microwave oven (CEM MARS 5Xpress) at 160 degrees for 30 min. The digested contents were passed through a 0.45 μm injector tip filter (Whatman Syringe Filters, 0.45 μm) and transferred to 15 mL plastic tubes. Faecal lead levels were measured using a graphite furnace atomic absorption spectrometer (THERMO SCIENTIFIC ICE 3400 AA), with a limit of detection for lead in stool of 0.1 μg/g.
Statistical analysisWe analysed the data with the software SPSS version 21.0 for Windows. We have expressed the results as mean and standard deviation (SD) or median and range. We used the Kolmogorov–Smirnov test to assess normality in the data distribution. Since the distributions of the BLL and FLL values were not normal (Kolmogorov-Smirnov test, P < .05), we described them using the median and interquartile range (IQR), comparing groups with the Mann-Whitney U test. The age, height, weight, haemoglobin (Hb), haematocrit (Hct) and ESR followed a normal distribution (P > .05) and were compared with the independent samples t test. We made intragroup comparisons with the Kruskal–Wallis test, and post hoc comparisons with Tamhane’s T2 test. We assessed the association between variables by means of the Spearman correlation test. We considered p-values of less than 0.05 statistically significant.
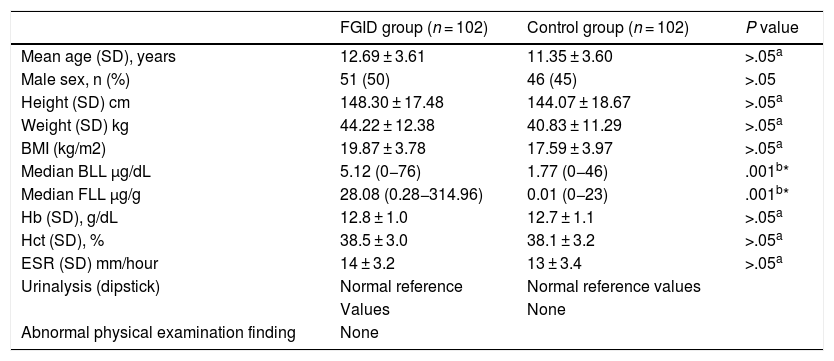
ResultsIn the group of 102 children with FGIDs, 51 (50%) were male, and the mean age was 12.69 ± 3.61 years, while in the group of 102 controls, 46 (45%) were male and the mean age was 11.35 ± 3.60 years. There were no statistically significant differences between the case and control groups in age, sex, height, weight, body mass index (BMI), or Hb, Hct or ESR values. The median BLL in the FGID group was significantly higher compared to the control group (5.12 vs 1.77 µg/dL; P < .001) (Table 1). The FGID group was subdivided into FC (n = 36; 35%), FAP (n = 36; 35%) and FN (n = 30; 29%).
Characteristics of children with functional gastrointestinal disorders and controls.
| FGID group (n = 102) | Control group (n = 102) | P value | |
|---|---|---|---|
| Mean age (SD), years | 12.69 ± 3.61 | 11.35 ± 3.60 | >.05a |
| Male sex, n (%) | 51 (50) | 46 (45) | >.05 |
| Height (SD) cm | 148.30 ± 17.48 | 144.07 ± 18.67 | >.05a |
| Weight (SD) kg | 44.22 ± 12.38 | 40.83 ± 11.29 | >.05a |
| BMI (kg/m2) | 19.87 ± 3.78 | 17.59 ± 3.97 | >.05a |
| Median BLL µg/dL | 5.12 (0−76) | 1.77 (0−46) | .001b* |
| Median FLL µg/g | 28.08 (0.28−314.96) | 0.01 (0−23) | .001b* |
| Hb (SD), g/dL | 12.8 ± 1.0 | 12.7 ± 1.1 | >.05a |
| Hct (SD), % | 38.5 ± 3.0 | 38.1 ± 3.2 | >.05a |
| ESR (SD) mm/hour | 14 ± 3.2 | 13 ± 3.4 | >.05a |
| Urinalysis (dipstick) | Normal reference | Normal reference values | |
| Values | None | ||
| Abnormal physical examination finding | None |
BLL, blood lead level; BMI, body mass index; ESR, erythrocyte sedimentation rate, FGID, functional gastrointestinal disorder; FLL, faecal lead level; Hb, haemoglobin; Hct, haematocrit; SD, standard deviation.
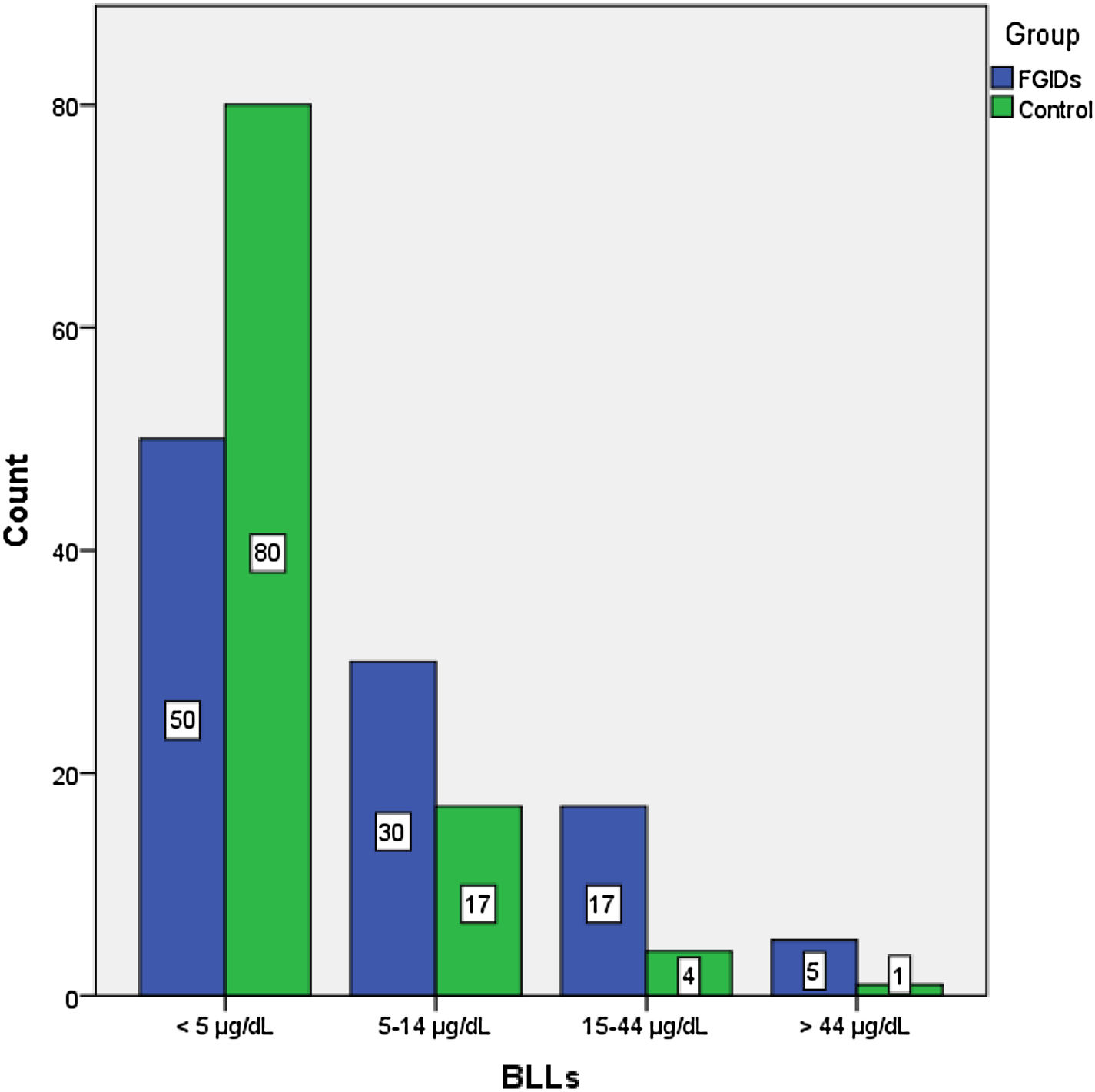
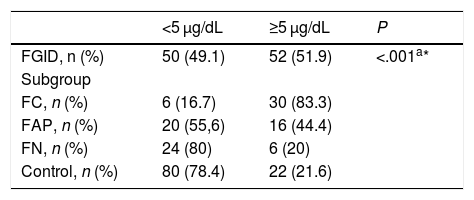
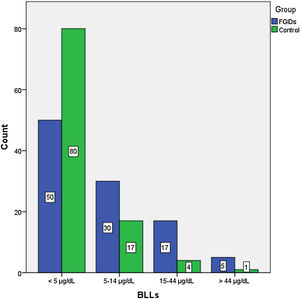
The BLLs of participants were classified into 4 categories: less than 5 µg/dL, 5–14 µg/dL, 15–44 µg/dL and more than 45 µg/dL (Fig. 1). We found the highest BLLs (76 µg/dL) in the FC subgroup. The BLLs were above 5 µg/dL in 51.9% of children with FGIDs and in 21.6% of controls (Table 2).
Frequency of low (<5 µg/dL) and high (≥5 µg/dL) blood lead levels in the functional gastrointestinal disorder and control groups.
| <5 μg/dL | ≥5 μg/dL | P | |
|---|---|---|---|
| FGID, n (%) | 50 (49.1) | 52 (51.9) | <.001a* |
| Subgroup | |||
| FC, n (%) | 6 (16.7) | 30 (83.3) | |
| FAP, n (%) | 20 (55,6) | 16 (44.4) | |
| FN, n (%) | 24 (80) | 6 (20) | |
| Control, n (%) | 80 (78.4) | 22 (21.6) |
FAP, functional abdominal pain; FC, functional constipation; FGID, functional gastrointestinal disorder; FN, functional nausea.
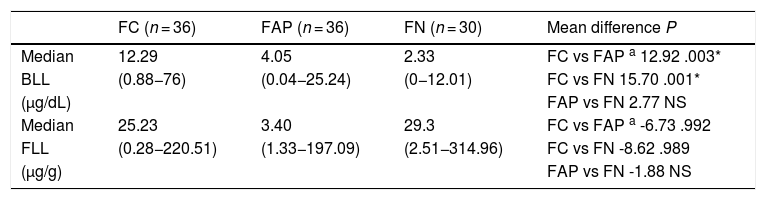
The median FLL in the FGID group was significantly higher compared to the control group (28.08 vs 0.01 μg/g; P = .001) (Table 1). Table 3 presents the median BLLs and FLLs in the FGID subgroups.
Blood and faecal lead levels in functional gastrointestinal disorder subgroups.
| FC (n = 36) | FAP (n = 36) | FN (n = 30) | Mean difference P | |
|---|---|---|---|---|
| Median | 12.29 | 4.05 | 2.33 | FC vs FAP a 12.92 .003* |
| BLL | (0.88−76) | (0.04−25.24) | (0−12.01) | FC vs FN 15.70 .001* |
| (µg/dL) | FAP vs FN 2.77 NS | |||
| Median | 25.23 | 3.40 | 29.3 | FC vs FAP a -6.73 .992 |
| FLL | (0.28−220.51) | (1.33−197.09) | (2.51−314.96) | FC vs FN -8.62 .989 |
| (µg/g) | FAP vs FN -1.88 NS |
BLLs, blood lead level; FAP, functional abdominal pain; FC, functional constipation; FLL, faecal lead level; FN, functional nausea; NS, not significant.
We divided the FGID and control groups into 2 age subgroups. In the FGID group, 37 participants were aged 4–11 years (36%) and 65 were aged 12–18 years (63%). There were no statistically significant differences between the 2 age subgroups in the FGID group in the median BLL (5.30 vs 4.87 µg/dL; P = .799) or the median FLL (26.03 vs 34.00 µg/g; P = .148). In the control group, 52 children were aged 4–11 years (53%) and 50 were aged 12–18 years (47%). We did not find statistically significant differences between the 2 age subgroups in the median BLL (2.30 vs 1.45 µg/dL; P = .348) or the median FLL (0.01 vs 0.01 µg/g; P = .08).
When we compared the BLLs in the 3 FGID subgroups, the Kruskal–Wallis and post hoc Tamhane T2 tests showed significant differences between the FC subgroup and the FAP and FN subgroups (P = .003 and P = .001, respectively). When it came to the FLL, we found no significant differences between subgroups (FC vs FAP, P = .992; FC vs FN, P = .989). We did not find a significant correlation between BLLs and FLLs in children with FGIDs (P = .123) or in any of the FGID subgroups (P > .05) (Table 4). In addition, there was no significant relationship between BMI and BLLs in either the FGID group (P = .492) or the control group (P = .732).
DiscussionIn our study, children with FGIDs had higher BLLs and FLLs compared to controls, and we found BLLs equal to or greater than 5 μg/dL in 51.9% of children with FGIDs and 21.3% of controls.
Functional gastrointestinal disorders, more recently referred to as disorders of gut-brain interaction, can affect any part of the GI tract. Although the prevalence of the different FGIDs varies over time, the most common FGID in children is FC.2 The findings of a detailed history and physical examination and symptom-based criteria suffice to diagnose FC, and further evaluation of potential organic causes of constipation may not be necessary.16 In case of an atypical history or abnormal findings of the physical examination in children with constipation, a comprehensive battery of laboratory tests is required for diagnosis.17 The pathogenesis of constipation due to lead toxicity is not fully understood. Lead is thought to prolong bowel transit time by reducing the number of neurons and disrupting the coordination of the autonomic nervous system.10,18
Few authors have investigated BLLs in children with constipation. Maleknejad et al. conducted a study in 90 Iranian children suffering from chronic constipation. They reported that the BLLs of children with chronic constipation were significantly higher compared to those of controls (11.643 vs 4.924 µg/dL).14 Zamani et al. studied 100 children aged 4 days to 12 years and found that 19% of the children with high BLLs had constipation.19 In contrast, in a study conducted in the United States by Raghu et al., only 1.36% of 441 children with constipation had high BLLs, and the authors found no correlation between constipation and BLLs.20 None of authors in previous studies shared information about whether children with FC were included in the study. In our study, we found significantly higher median BLLs in the FC subgroup compared to the FAP and FN subgroups (12.29, 4.06 and 2.33 µg/dL, respectively). Moreover, we found that 83.3% of the 36 children in the FC subgroup had BLLs of 5 μg/dL or higher. These findings suggest that higher lead levels in children with FC may result from a prolonged bowel transit time. Our findings contrast with those of Raghu et al., which may be explained by the larger sample in their study (n = 441), and differences in the environments where the children resided.
Abdominal discomfort related to lead may be present with BLLs as low as 10–30 μg/dL; however, colic is typically associated with lead levels greater than 30 µg/dL.21 The exact mechanisms underlying lead-induced abdominal pain remain unclear. One possible mechanism is the overproduction of aminolevulinic acid, changes in visceral smooth muscle tone and impaired gastrointestinal motility.22 Some studies have emphasized that there is an association between elevated BLLs and abdominal pain. Ataee et al. analysed the BLLs of children suffering from chronic non-organic gastrointestinal disorders and found that BLLs in children with chronic non-organic abdominal pain and/or constipation were higher compared to the control group (4.34 vs 1.39 µg/dL).23 Recently, Zhang et al. measured BLLs in 2271 children aged 0–7 years in Chengdu, and concluded that assessment of lead levels in children with abdominal pain may be useful in identifying children with high BLLs.24 In our study, we found that the median BLL in the FAP subgroup was 4.05 µg/dL (range, 0.04−25.24) and that 44.4% of the 36 children in the FC subgroup had a BLL of 5 μg/dL or higher. None of the children with FAP had a BLL of 30 μg/dL or greater, and none had colic.
Functional nausea is relatively less common than both FC and FAP. The exact cause of FN, as is the case of FC and FAP, is not well known. It is believed there several factors are at play, involving the viscera and central nervous system, mechanical triggers, psychosocial stress and dietary habits. Lead has long been known to cause nausea. There are some theories regarding how lead can cause nausea. Most of them focus on the paralyzing effect of lead on the GI tract. According to these theories, lead would trigger nausea by delaying gastric emptying and reducing gastric tonus.10,25 So far, no comprehensive studies have been published that evaluate the relationship between BLLs and FN. In addition, only 1 case was reported in children by Begovic et al. The case occurred in a boy aged 16 years presenting with nausea and was found to be caused by gastric dilation secondary to chronic lead poisoning; the BLL in this patient was 30 μg/dL.10 We found a significantly lower median BLL in the FN subgroup compared to the FC subgroup (2.33 vs 12.29 µg/dL). In addition, 20% of the 30 children in the FN subgroup had BLLs of 5 μg/dL or higher. Although the case described by Begovic et al. supports the hypothesis that lead can cause nausea by paralyzing the gastrointestinal tract, our findings do not support it, as 80% of children with FN had BLLs of less than 5 μg/dL and none had features compatible with gastric dilation.
Since lead in the body is eliminated primarily through the urine, hair, nails, sweat and faeces, numerous studies have analysed measurements of lead concentrations in body fluids, nails and faeces. Schouw et al. conducted a study in healthy adults aged 20–30 years and found faecal concentrations of lead of 0.12 to 0.27 g/kg.26 Yabe et al. investigated lead and cadmium excretion in faeces and urine in children residing in polluted townships in Zambia. They found faecal lead concentrations in these children ranging from 2.27 to 2252 mg/kg (dry weight) and proposed that faecal and urine lead levels may be useful for monitoring lead exposure in children.27 In our study, we found a median FLL in children with FGIDs of 28.08 µg/g, equivalent to 28.08 mg/kg, with a range of 0.28–314.96 µg/g (dry weight). There were no statistically significant differences in FLLs between subgroups of children with FGIDs. We did not find any studies in the paediatric literature that established faecal lead reference levels or analysed the excretion of lead in faeces in different FGIDs. In consequence, we could only compare our results with those of Yabe et al. The range of FLLs in their study was greater compared to the range found in ours. One possible explanation is that Yabe et al. conducted their study in areas with high environmental pollution.
Several studies conducted in multiple parts of the world demonstrate that BLLs are higher in children living close to industrial areas and in cities with air pollution. A study by Lin et al. found that 96% of Chinese children living in polluted villages had BLLs higher than 10 μg/dL.28 Recently, Dhimal et al. reported that 64.4% of children living in the Kathmandu Valley of Nepal had high BLLs exceeding the 5 μg/dL threshold.29 In our study, we found that 21.6 % of healthy controls had BLLs of 5 μg/dL or higher. We think that the reason for the high BLLs observed in healthy children may be the presence of iron and steel manufacturing and moderate air pollution in the province of Karabük, which is surrounded by mountains.
In this study, we did not find a significant correlation between BLLs and FLLs in children with FGIDs (r = –0.154; P = .123). In contrast, we detected a strong positive correlation between BLLs and FLLs in the control group (r = 0.432; P = .001). Only a few studies have investigated the relationship between BLLs and faecal lead excretion in children. Hammond et al. conducted a study in healthy children exposed to lead through paint in the home environment and found a positive correlation between BLLs and faecal lead excretion.30 As mentioned above, Yabe et al. also investigated lead excretion in faeces and reported a positive correlation between BLLs and faecal lead excretion in children from polluted townships.27 The main feature in both studies was the investigation of children exposed to environmental pollutants. The finding of these studies differed from the findings in our sample. This may be due to the fact that the studies of the other authors were not conducted in children with FGIDs.
There are limitations to our study. First, no studies to date have analysed BLLs and FLLs in children with FGIDs, so we could only compare our findings to a small number of previous studies. Second, we were unable to determine the main source of lead exposure in children with high BLLs. In light of the data obtained in our study, we notified the local agency of occupational safety and health so it could determine the main sources of lead exposure. Third, we did not find a correlation between BLLs and faecal lead excretion in the FGID group, although we did find a positive correlation between them in the control group. In order to understand this difference, it may be helpful to determine and compare lead levels in other specimens, such as urine, sweat and hair, but we were unable to do so due to budget constraints.
In conclusion, no studies to date have analysed both BLLs and FLLs in children with FGIDs. Our results demonstrated that children with FGIDs had significantly higher BLLs and FLLs compared to healthy controls. In addition, our study highlighted that lead toxicity was common and frequently mimicked gastrointestinal disorders in children. Therefore, we think clinicians should be alert to the possibility of lead exposure in the diagnosis of FGIDs.
Conflict of InterestNone declared.
Please cite this article as: Sevinc N, Bilici N, Sevinc E, Dogan E. Niveles séricos y fecales de plomo en niños con distintos trastornos digestivos funcionales. An Pediatr (Barc). 2022;96:35–42.
Previous presentation: This study was presented as poster at the 6th World Congress of Pediatric Gastroenterology, Hepatology and Nutrition (WCPGHAN 2020), June 3–6, 2020, Copenhagen, Denmark.