Post-prandial glucose control is essential to achieve metabolic goals in patients with type 1 diabetes mellitus (T1DM). The new “faster aspart” insulin has a pharmacological profile noted for its faster absorption and onset of action, and increased early availability, resulting in improved blood glucose control after meals. The main objective of the study was to analyse the efficacy of “faster aspart” vs. “insulin aspart” in children and adolescents with DM1 on sensor-augmented pump treatment.
Patients and methodsMulticentre, longitudinal and prospective analytical trial evaluating the use of faster aspart insulin for three months in children with T1DM with MiniMed640G® sensor-augmented pumps previously treated with aspart insulin. At the beginning and end of the study the following variables were analysed for subsequent comparison: mean sensor glucose, percentage of time in range, hypoglycaemia and hyperglycaemia, area under the curve (AUC) <70 and >180 mg/dL, mean sensor glucose pre- and postprandial in main meals, daily insulin requirements, basal/bolus percentage, and HbA1c. Acute complications, adverse events and satisfaction survey were assessed.
ResultsThe study included 32 patients with a mean of 13.49 ± 2.42 years of age and with T1DM of 7.0 ± 3.67 years of onset. The use of faster aspart was associated with lower time in hyperglycaemia >180 mg/dL (25.8 ± 11.3 vs. 22.4 ± 9.5; p = .011) and >250 mg/dL (5.2±4.9 vs. 4.0 ± 3.6; p = .04), lower AUC >180 mg/dL (10.8 ± 6.5 vs. 9.3 ± 6.1; p = .03), and increased time in range (71.4 ± 10.0 vs. 74.3 ± 9.2; p = .03). No significant changes in hypoglycaemia, HbA1c, insulin requirements, and basal/bolus percentages were detected. Faster aspart was safe and well-evaluated by patients and caregivers.
ConclusionsFaster aspart achieves better glycaemic control by increasing glucose time in range in children and adolescents with T1DM on treatment with sensor-augmented pumps.
El control glucémico postprandial es fundamental para conseguir los objetivos metabólicos en pacientes con diabetes mellitus tipo 1 (DM1). La nueva insulina faster aspart presenta un perfil farmacológico caracterizado por una absorción e inicio de acción más rápidos, mayor disponibilidad precoz y menor incremento de la glucosa postprandial. El objetivo principal del estudio fue analizar su eficacia en pacientes con DM1 tratados con un sistema integrado.
Pacientes y métodosEstudio analítico, longitudinal, prospectivo y multicéntrico, evaluando el empleo de faster aspart durante tres meses en pacientes en edad pediátrica con DM1 con sistema integrado MiniMed640G® tratados previamente con insulina aspart. Al inicio y final del estudio se analizaron para posterior comparación: glucosa media, porcentajes de tiempo en objetivo, tiempo en hipoglucemia e hiperglucemia, área bajo la curva (AUC) <70 y >180 mg/dL, glucosa media pre y postprandial en comidas principales, necesidades diarias de insulina, porcentaje basal/bolo y HbA1c. Se registraron complicaciones agudas y eventos adversos, y se evaluó grado de satisfacción mediante encuesta.
ResultadosSe incluyeron 32 pacientes de 13,49 ± 2,42 años de edad con DM1 de 7,0 ± 3,67 años de evolución. Faster aspart se asoció a menor porcentaje de tiempo en hiperglucemia >180 mg/dL (25,8 ± 11,3 vs. 22,4 ± 9,5; p = 0,011) y >250 mg/dL (5,2 ± 4,9 vs. 4,0 ± 3,6; p = 0,04) y AUC >180 mg/dL (10,8 ± 6,5 vs. 9,3 ± 6,1; p = 0,03), incrementándose el tiempo en objetivo (71,4 ± 10,0 vs. 74,3 ± 9,2; p = 0,03) sin aumentar hipoglucemias. Las necesidades de insulina, porcentajes basal/bolo y HbA1c no se modifican significativamente. Faster aspart fue bien tolerada y valorada por los participantes.
ConclusionesFaster aspart consigue un mejor control glucémico, aumentando el tiempo de glucosa en objetivo en niños y adolescentes con DM1 en tratamiento con un sistema integrado.
Type 1 diabetes (T1D) is characterised by fasting and post-prandial hyperglycaemia secondary to deficient insulin levels due to the autoimmune destruction of insulin-producing pancreatic beta cells. The results of the Diabetes Control and Complication Trial (DCCT)1,2 and, later on, the Epidemiology of Diabetes Interventions and Complications (EDIC) study3 evinced the importance of maintaining blood glucose levels near the normal range to prevent microvascular complications in individuals with T1D. From that moment, the goal of treatment has been to achieve blood glucose and glycated haemoglobin (HbA1c) levels as near the normal range as possible from the onset of disease. However, changes in weight, body composition and insulin sensitivity complicate treatment in children and adolescents, so that only a minority achieve the target blood glucose values recommended by scientific societies.4,5
At present, the standard of care is intensive basal-bolus insulin therapy to remedy physiological pancreatic production through multiple daily injections (MDI approach) or continuous subcutaneous insulin infusion (CSII).6 The use of CSII has been growing in the past 2 decades,7 as it has been shown to improve glycaemic control and decrease the risk of severe hypoglycaemia compared to the MDI approach.8
The control of postprandial blood sugar is essential for achieving the HbA1c values recommended by scientific societies,9,10 especially in patients with good overall control.11 In addition, data from several epidemiological studies suggest that postprandial hyperglycaemia is associated with increases in cardiovascular risk and mortality,12,13 although the findings of experimental studies on patients have been inconclusive.14,15 In healthy individuals, seeing food and starting to eat stimulate a quick release of insulin that inhibits glucagon secretion (and therefore its stimulation of hepatic glucose output) and promotes peripheral glucose uptake, thereby limiting the postprandial increase in blood glucose levels. This complex and sophisticated regulatory system is altered in individuals with diabetes, leading to higher postprandial increases in glucose levels. Thus, the ideal type of insulin to use in boluses would be a rapid-acting insulin with a quick peak and a very short duration of action.
Compared to regular insulin, rapid-acting insulin analogues (RAIAs) (insulin lispro, aspart and glulisine) have a more rapid onset (10-15 minutes) and a shorter duration of action. However, despite advances in their formulation, RAIAs still reach the peak too slowly to control postprandial hyperglycaemia and suppress hepatic glucose output,16,17 and also exhibit a longer duration of action compared to endogenous insulin.
In patients that carry sensor-augmented pumps (an insulin pump combined with a continuous interstitial glucose monitor), delays in the absorption and action of RAIAs, combined with the time lag between the interstitial glucose readings made by the sensor and the adaptive algorithm, are one of the greatest challenges in achieving effective closed-loop control (artificial pancreas).18
The new fast-acting insulin aspart (faster aspart) is a novel formulation of insulin aspart that contains 2 additional excipients: nicotinamide (vitamin B3) and L-arginine. Nicotinamide is responsible for faster absorption by increasing the initial abundance of insulin aspart monomers in the subcutaneous depot and producing a transient local vasodilatory effect.19 L-arginine serves as a stabilizing agent. Pharmacokinetic and pharmacodynamic studies have found that faster aspart appears in the bloodstream twice as fast, achieving an exposure to insulin in the first 30 minutes from administration that is twice as large and an early glucose-lowering effect that was 74% greater.20 In addition, although the peak concentration and total exposure to insulin did not differ, the offset of exposure occurred 12 to 14 minutes earlier with faster apart than with conventional insulin aspart. The variability in the action of faster aspart, both within and between individuals, is low and comparable to that of insulin aspart. Studies in children and adolescents with T1D had similar outcomes.21 The absorption profile of faster aspart improves even more when administered as CSII instead of through MDI, as it mimics the physiological secretion of insulin better. Early exposure to faster aspart is 3 times greater and its glucose-lowering effect approximately 100% greater in the first 30 minutes, and the offset of the glucose-lowering effect occurs 24 minutes earlier compared to insulin aspart.22 In children and adolescents, preprandial administration of faster aspart was associated with a significant reduction in postprandial hyperglycaemic excursions compared to administration of insulin aspart, without an increase in associated risks.23 The same study found that when it came to the HbA1c concentration, postprandial use of faster aspart was not inferior to insulin aspart, while preprandial use of faster aspart was associated with a significantly lower HbA1c concentration.
The main aim of our study was to assess the impact of faster aspart on glycaemic control in children and adolescents with T1D managed with sensor-augmented pump therapy.
Study sample and designStudy designWe conducted a prospective, longitudinal analytical study between July and October 2019 in patients with T1D managed by the child and adolescent diabetes units of the Hospital San Pedro de Alcántara (Caceres, Spain) and Hospital Virgen del Puerto (Plasencia, Spain).
ParticipantsThe inclusion criteria were the following: paediatric patient, diagnosis of T1D, duration of disease longer than 1 year, previous treatment lasting a minimum of 3 months with the MiniMed 640G-SmartGuard® sensor-augmented insulin pump (Medtronic®, Northridge, CA, USA) and insulin aspart. We obtained written informed consent from parents and assent from patients aged 12 or more years. At the time of recruitment, faster aspart was only indicated in patients aged more than 18 years with type 1 or type 2 diabetes, although the trials for marketing authorization in the paediatric age group had already been completed and the extension of the indication to children aged at least 1 year was imminent. For this reason, we also obtained informed consent for the use of faster aspart.
The MiniMed640G-SmartGuard® sensor-augmented pump automatically discontinues insulin infusion whenever a descent in the interstitial glucose level to down to 20 mg/dL above the established limit is expected to happen in the next 30 minutes as long as the current glucose level is 70 mg/dL or less above said threshold, with the duration of the suspension ranging from 30 minutes to 2 hours. Insulin infusion resumes automatically when the interstitial glucose level is at least 20 mg/dL above the set threshold and the system predicts that the level will increase to 40 mg/dL above the threshold in the next 30 minutes; the patient can also manually resume the infusion if desired. Patients whose pumps did not have the predictive low-glucose discontinuation function on when the study started kept it off for the 3 months that followed to keep modifications in this setting from contributing to the differences observed during the study. Real-time continuous glucose monitoring (CGM) was carried out with the Guardian®Sensor 3 and the Guardian®Link 3 transmitter (Medtronic®).
MethodsWe started by downloading the data recorded by the pumps in the past month using the CareLink® software (Medtronic®): CGM sensor duration, mean interstitial glucose readings in the past month, coefficient of variation (CV), mean interstitial glucose before and 2 hours after the main meals, percentage of time in target range (70-180 mg/dL), below target/in hypoglycaemia (< 70 and < 54 mg/dL) and above target/in hyperglycaemia (> 180 y > 250 mg/dL), area under the curve (AUC) below target (< 70 mg/dL) and above target (> 180 mg/dL), daily insulin use and basal/bolus balance. We defined the limits for the time in range, hyperglycaemia and hypoglycaemia based on international consensus guidelines.24 We also collected anthropometric data (body mass index [BMI],25 pubertal development) and measured HbA1c concentrations (Afinion 2®, Abbott).
During the 3 months of follow-up, patients used faster aspart in their insulin pumps. At the start of the study, we informed families of the time-actin profile of the novel insulin formulation and the likely need to reduce waiting times between bolus administration and initiation of food intake. We also informed them of the potential need to change pump settings based on the blood glucose profiles observed with the use of faster aspart. Having provided this information, we maintained the usual schedule of visits for patient monitoring in the clinic, without engaging in closer follow-up or education for the purpose of the study. Patients continued to use the information provided by the sensor and making adjustments to the pump based on the trend arrows as they had before and had learned in previous educational interventions, with no new recommendations provided for the purpose.
At the end of the study, we collected data on the same variables documented at baseline, in addition to information on reported problems and detected adverse events. We also asked parents and patients to participate in a satisfaction survey in which they rated their satisfaction with the new insulin on a scale from 1 to 5 (1 = very dissatisfactory, 2 = dissatisfactory, 3 = acceptable, 4 = satisfactory, 5 = very satisfactory) and expressed whether or not they wished to continue using it.
Statistical analysisThe statistical analysis of the data was performed with the Statistical Package for the Social Sciences (SPSS®), version 24 for Windows® (IBM SPSS Statistics, Armonk, NY, USA). We compared continuous data by means of the paired sample t-test for in case of a normal distribution and the Wilcoxon test otherwise. For every test, we defined statistical significance as a p-value of less than 0.05.
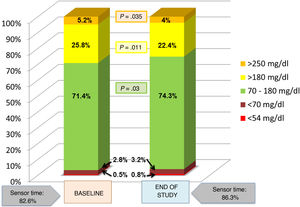
ResultsOf the total of 45 patients that met the inclusion criteria, 32 agreed to participate (17 female and 15 male), of who 81% had reached puberty. The mean age of the sample was 13.49 ± 2.42 years, and the mean duration of disease 7.0 ± 3.67 years. The mean time elapsed from onset of T1D to initiation of treatment with CSII was 2.23 ± 2.57 years. Table 1 presents the rest of the baseline characteristics of the participants. Six patients that had not set up the predictive low-glucose suspension function in their pumps kept this function off throughout the study, relying on the alarms that were already set at baseline. One female participant withdrew from the study due to abdominal pain not temporally associated with bolus infusion. The average CGM sensor duration was 82.6% at baseline and 86.3% at the end of the study.
Baseline characteristics of the sample.
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 13.49 ± 2.42 | (7.07-17.27) |
| T1D disease duration (years) | 7.0 ± 3.67 | (1.94-15.83) |
| Age at initiation of CSII (years) | 8.71 ± 3.81 | (0.95-14.07) |
| BMI z-score | 0.05 ± 1.00 | (-1.36-2.03) |
| Insulin requirements (IU/kg/day) | 0.83 ± 0.16 | (0.45-1.09) |
| Basal (%) | 43.72 ± 8.27 | (29-59) |
| Bolus (%) | 56.47 ± 8.47 | (41-71) |
| HbA1c (%) | 6.95 ± 0.54 | (5.7-8.4) |
| Number capillary blood glucose readings/day | 5.91 ± 1.40 | (2.5-8.3) |
| Mean interstitial glucose (mg/dL) | 148.69 ± 15.97 | (113-196) |
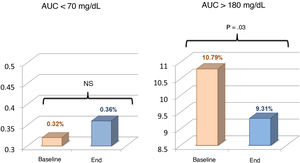
The comparison of data obtained at baseline and in the last month of the study during use of faster aspart revealed a significant decrease in the mean interstitial glucose level from 149 ± 16 to 145 ± 14 mg/dL (P = .04). The time in range (70-180 mg/dL) increased from 71.4% ± 10.0% to 74.3% ± 9.2% (P = .03). There was a reduction in the percentage of time spent below range at levels of less than 180 mg/dL (25.8% ± 11.3% vs 22.4% ± 9.5%; P = .011) and less than 250 mg/dL (5.2% ± 4.9% vs. 4.0% ± 3.6%; P = .035). The AUC for glucose levels under 180 mg/dL also decreased significantly from 10.8 ± 6.5 to 9.3 ± 6.1 (P = .03). The time in hypoglycaemia (below both 70 and 54 mg/dL) and the AUC for glucose levels under 70 mg/dL increased with the switch to faster aspart, but the differences were not statistically significant (Figs. 1 and 2), both in the overall sample and in the separate analysis of patients that had the predictive low-glucose discontinuation function on versus off during the follow-up. The comparison of these 2 groups did not evince significant differences at baseline or at the end of the study in the time spent in hypoglycaemia. The CV did not change significantly (34.5 vs 35.1; P > .03). We observed a decreasing trend in the concentration of HbA1c (from 6.95% to 6.85%), although this difference was also not statistically significant. There were no episodes of severe hypoglycaemia or acute ketoacidosis during the follow-up.
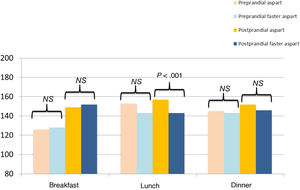
Comparing the mean interstitial glucose before and 2 hours after the main meals (breakfast, lunch and dinner) we only found a significant decrease in postprandial glucose after lunch (156.9 ± 22.0 vs 143.0 ± 23.9; P < .001). We did not find significant differences between preprandial and postprandial levels at breakfast or dinner (Fig. 3).
Insulin requirements adjusted for weight and the basal/bolus balance did not change significantly after the change in insulin. There were also no significant changes in the BMI of the patients.
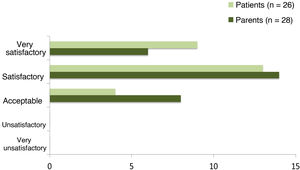
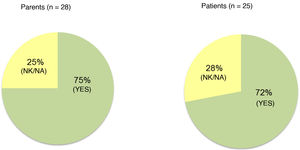
Out of the 31 families that completed the follow-up, we obtained responses to the satisfaction survey from 28 caregivers and 26 patients (4 of the 5 patients that did not respond were not mature enough to do it). Of these respondents, 85% of patients and 71% of caregivers reported feeling satisfied or very satisfied with faster aspart (Fig. 4). When asked about whether they would like to continue using the new insulin, 75% of parents and 72% of patients answered that they did (Fig. 5). The 31 participants that completed the follow-up continued using faster aspart in their pumps.
The drawback/adverse effect described most frequently was the sensation of mild pain or itching during bolus infusion. Table 2 details every comment made on the subject.
Adverse events reported by participants.
| Adverse events |
|---|
| Mild pain or burning sensation with bolus administration (n = 9). |
| Hypoglycaemic excursions (n = 8). |
| Air bubble formation with reservoir loading (n = 4). |
| Greater initial glycaemic instability (n = 3). |
| Decreased effectiveness of correction boluses (n = 2). |
| Pump occlusion /infusion site saturation (n = 1). |
Developed with the aim of better mimicking the pancreatic response to food intake, the pharmacological profile of faster aspart has proven more effective for control of postprandial blood glucose levels in previous studies.26,27 Since faster aspart has only been recently authorised for use in paediatric patients from age 1 year, the evidence of its use in children and adolescents in general and in paediatric patients managed with sensor-augmented pumps in particular is still scarce. To our knowledge, this is the first prospective study conducted in Spain that has assessed the use of faster aspart in paediatric patients with T1D managed with sensor-augmented pumps with the described methods under real-world conditions as opposed to a controlled clinical setting.
The findings of the study suggest that the accelerated absorption kinetics of faster aspart compared to insulin aspart led to a significant improve in glycaemic control in patients treated with sensor-augmented pumps, increasing the time in range and decreasing the time above range without a significant increase in the time below range. Given the pharmacological profile of faster aspart, it would be fair to assume that the decrease in the time spent in hyperglycaemia is due to improved control of postprandial excursions. It has been established that in patients with good overall glycaemic control, as was the case of participants in our study, improvement is associated with better control of postprandial hyperglycaemia.11 However, we only found evidence of significant improvement in postprandial glucose levels after the main meal of the day. This could be due to the fact that the pump data downloaded by the software for analysis of postprandial glucose corresponded to 2 hours after bolus delivery. Since the availability of insulin delivered by CSII is approximately 3 times greater in the first 30 minutes using faster aspart compared to insulin aspart,28 it would have been preferable to use data from the earlier postprandial period (1 hour after bolus delivery), as it may have revealed greater differences and explained the decrease in the time in hyperglycaemia to a greater extent. Other studies in both paediatric and adult patients23,27 have found smaller increases in interstitial glucose levels 1 and 2 hours after bolus delivery with the use of faster aspart compared to insulin aspart.
The concentration of HbA1c decreased slightly with the use of faster aspart, although not significantly. Based on the findings of previous studies, a 10% increase in the time in range (2.4 hours/day) is associated with a decrease in HbA1c of approximately 0.6%.29,30 In our study, the use of faster aspart was associated with a modest but significant increase in the time in range of nearly 3%, which could explain the observed decreasing trend in HbA1c (0.1%).
The ONSET 5 study27 compared the efficacy of faster aspart and insulin aspart in 472 adults with T1D treated with CSII over a period of 16 weeks. However, for reasons that remained unclear, the reduction in HbA1c was larger in the group treated with insulin aspart even though postprandial glycaemic control was better in the faster aspart group. The need to adjust basal and bolus insulin delivery after the switch to faster aspart may explain this discrepancy.31 Although the dose conversion applied to the switch from insulin aspart to faster aspart is 1:1,32 well-defined consensus-based recommendations for the use of faster aspart in CSII therapy have yet to be published. In our study, we did not find differences in the total daily dose of insulin adjusted for weight nor the basal/bolus balance after the switch from insulin aspart to faster aspart. The ONSET 7 study,23 also in the paediatric population, did not find differences either, although in the ONSET study treatment was delivered through MDI. In any case, as noted by other authors based on real-world clinical practice,31 it is important to emphasise the need to revise the pump settings on switching to faster aspart, which is facilitated considerably by CGM and adequate knowledge of the nutrient composition and glycaemic index of different foods. Similarly, the use of alternative bolus delivery speeds should be considered, as delivery speed could have an impact on active insulin time.33
The early postprandial period is generally characterised by a low incidence of hypoglycaemic excursions, yet some studies have found a higher risk of hypoglycaemia between 1 and 2 hours post meal with faster aspart.26,27 We analysed postprandial glucose levels 2 hours after bolus administration and did not differentiate between the early and late postprandial periods. In the comments collected from participants at the end of the study, 8 patients reported postprandial hypoglycaemic episodes or a rapid drop in interstitial glucose levels after bolus delivery. Given the quicker absorption of faster aspart, it seems prudent to reassess the time patients should wait from bolus administration to starting the meal and assess the need to make adjustments to boluses to avoid hypoglycaemia in the early postprandial period in case food intake is delayed, the meal has a high fat content or gastroparesis.32 On the other hand, finetuning the timing of faster aspart delivery to improve the overlap of its action and the postprandial elevation in blood glucose would reduce the risk of late hypoglycaemic excursions (at 3 or 4 hours) observed with the use of RAIAs,34 although we did not assess this aspect in our study. We ought to mention that some of the studies done in the past measured postprandial blood glucose following ingestion of a standardized mixed liquid meal.26,27 However, in studies like the one presented here assess real-world experiences, the optimal time to assess postprandial blood glucose levels may be debatable.
Interestingly, the comparative analysis of patients that used the predictive low-glucose discontinuation function and patients that did not found no significant differences between the groups in the time spent in hypoglycaemia. It would be reasonable to expect longer times in the hypoglycaemic range in patients that did not have this function set up, both at baseline and at the end of the study, but we did not find evidence of such a difference, possibly because of the small sample size.
At the same time, it is also quite possible that the current algorithms and clinical practice guidelines based on CGM35,36 will need revising to make adjustments in line with the characteristics of faster aspart.
The adverse events reported by the end of the study corroborate the safety and good tolerability of faster aspart reported in previous studies.23,27,34 Pain or a burning sensation with bolus administration, the most frequent adverse event reported in our study, had already been described.31 Users also reported a lower-than-expected effectiveness of correction boluses, which leads us to reconsider the appropriateness of changing the active insulin time. Other problems reported by participants were the formation of bubbles on loading the reservoir and the necessity of changing the infusion set more frequently. The latter issue, reported by a single user, had already been described in the past,31 although a previous study found a similar compatibility of insulin aspart and faster aspart with CSII systems.37
Except for 1 patient that withdrew from the study due to abdominal pain (not associated with bolus delivery), all participants chose to continue using faster aspart after the conclusion of the 3-month follow-up. The possibility of reducing the wait time between food bolus administration and initiation of intake (especially in case of preprandial hyperglycaemia) is a great advantage, as real-world experience shows that patients frequently shorten or altogether skip this wait against recommendations.38 Faster aspart also improved control in patients with unpredictable eating habits that require bolus administration after meals (e.g., infants, picky eaters, or patients with intercurrent disease).
The design of our study limits the strength of our conclusions, as it was not a randomised controlled and double-blind trial. In addition, the sample was small and the study was performed in patients with good glycaemic control. Although it is likely that patients with poorer baseline control will exhibit a significant improvement after intervention,39 el our studies evinces that there is still room for improvement even in motivated patients with good glycaemic control.
In short, in agreement with previous evidence, the findings of our study support the potential benefit of faster aspart insulin in CSII therapy. Its use in children and adolescents with T1D and treated with sensor-augmented pumps was associated with a reduction in the mean interstitial glucose level, the duration and severity of hyperglycaemic excursions and an increased time in range without a significant increase in hypoglycaemic excursions. We found a decreasing (although not significant) trend in the HbA1c concentration. Although the need to make changes to pump settings after switching between insulins needs to be considered, we did not find significant differences in insulin requirements or the basal/bolus balance. Faster aspart insulin is safe and positively viewed. The current scarcity of real-world evidence calls for performance of additional studies to define the actual usefulness of faster aspart insulin and maximise its benefits.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: González de Buitrago Amigo J, González García A, Díaz Fernández P, Fernández Llamas M, Tejado Bravo ML, de Nicolás Jiménez JM, et al. Impacto de faster aspart sobre el control glucémico en niños y adolescentes con diabetes tipo 1 en tratamiento con un sistema integrado. An Pediatr. 2021;95:321–329.