Early identification of neonates exposed to drugs of abuse during pregnancy allows a more precise clinical management.
ObjectivesTo describe the clinical characteristics and to identify risk factors associated with the early detection of neonates exposed to drugs of abuse in a Neonatal Intermediate and Intensive Care Unit.
MethodsProspective observational study of neonates with and without clinical suspicion of prenatal exposure to drugs of abuse. Meconium was analyzed using standard chromatographic techniques. Univariate and multivariate statistical analyzes were performed.
Results372 neonates were included. Exposure to drugs of abuse was detected in 49 (13.2%) cases: in 41 (83.7%) one drug and in 8 (16.3%) more than one. Somatometry at birth revealed: a) lower length percentile in those exposed to some drug, more than one and cannabis; b) lower weight percentile in those exposed to cannabis and of these compared to those exposed to alcohol. In neonates older than 34 pregnancy weeks (PW): a) lower length percentile in those exposed to any substance; b) lower percentile of length and weight in exposed to more than one. The most clinically relevant independent risk factors useful to detect cases of prenatal exposure to drugs of abuse were (Odds ratio (95% CI)): reason for admission other than prematurity (5.52 (2.55-1.93)), length percentile less than 33 (1.95 (1.05-3.60) and 2.14 (1.04-3.40) in older than 34 PW) and social dystocia/uncontrolled pregnancy in older than 34 PW (4.47 (1.03-19.29)).
ConclusionsThere are somatometric alterations and risk factors that can help in the early detection of neonates exposed to drugs of abuse. The somatometric alterations identified can be useful to extend the differential diagnosis of these alterations and to study their causes.
La identificación temprana de los neonatos expuestos a drogas de abuso permite realizar un manejo clínico más preciso.
ObjetivosDescribir las características clínicas e identificar factores de riesgo asociados a la detección precoz de neonatos expuestos a drogas de abuso en una Unidad de Cuidados Intensivos e Intermedios Neonatales.
MétodosEstudio observacional prospectivo de neonatos con y sin sospecha clínica de exposición prenatal a drogas de abuso. Se analizó meconio empleando técnicas cromatográficas estandarizadas. Se realizaron análisis estadísticos univariante y multivariante.
ResultadosSe incluyeron 372 neonatos. En 49 (13,2%) casos se detectó exposición a alguna droga de abuso; en 41 (83,7%) a una, y en ocho (16,3%) a más de una. La somatometría al nacimiento objetivó: a) menor percentil de longitud en expuestos a alguna droga, a más de una y a cannabis; b) menor percentil de peso en expuestos a cannabis, y de éstos en comparación con los expuestos a alcohol. En mayores de 34 semanas de gestación (SG): a) menor percentil de longitud en expuestos a alguna droga; b) menor percentil de longitud y peso en expuestos a más de una. Los factores de riesgo independientes clínicamente útiles para detectar casos de exposición prenatal a drogas de abuso fueron (odds ratio [IC 95%]): motivo de ingreso distinto a prematuridad (5,52 [2,55-1,93]), percentil de longitud menor a 33 (1,95 [1,05-3,60]) y (2,14 [1,04-3,40]) en mayores de 34 SG y distocia social/embarazo no controlado en mayores a 34SG (4,47 [1,03-19,29]).
ConclusionesExisten alteraciones somatométricas y factores de riesgo que pueden ayudar a detectar precozmente a los neonatos expuestos a drogas de abuso. Las alteraciones somatométricas identificadas pueden servir para ampliar su diagnóstico diferencial y el estudio de sus causas.
Neonates that have been exposed to drugs of abuse during gestation are at higher risk of admission to a neonatal intensive care unit (NICU) or neonatal intermediate care unit (NIMCU). Their early identification allows performance of a suitable clinical evaluation, referral to early intervention services and management of withdrawal symptoms in the infant, if they develop.1 Testing of biological matrix samples for markers of drug and alcohol exposure is a useful tool to detect prenatal exposure in infants.2–5 This is particularly relevant if detection occurs during the hospital stay to guide decisions regarding health care and social services. It also sets the foundation for adequate medical and social follow-up in childhood and adolescence.6,7 Although the scientific evidence supports their use, these tests are still not widely used in clinical practice.
The clinical spectrum of prenatal drug exposure is broad, ranging from spontaneous miscarriage, intrauterine foetal demise, congenital malformations, low birth weight, preterm birth, foetal distress, premature rupture of membranes, asphyxia and ischaemic stroke to abnormal heart rhythms and respiratory patterns. It is also associated with neonatal abstinence syndrome and organic and neuropsychiatric disorders with onset in early childhood.6,7
In addition, exposure to alcohol during gestation can result in a constellation of physical, cognitive and behavioural deficits known as foetal alcohol spectrum disorder (FASD). Foetal alcohol syndrome (FAS) is the most severe form of this spectrum.8–10 Foetal alcohol spectrum disorder and FAS are the most frequent and easily preventable causes of acquired developmental disabilities in infants.
Estimating the prevalence of prenatal exposure to drugs of abuse is difficult given the heterogeneous results obtained on account of the different designs and methodology of the studies on the subject.6 Studies that used biomarkers in Spain and Italy reported a prevalence of prenatal drug exposure of 7.9% to 15.9% and of prenatal alcohol exposure of 1.7% to 45%.4,11–14
The aim of our study was to describe the clinical characteristics of infants with prenatal exposure to drugs of abuse and identify risk factors associated with the early detection of these patients in a neonatal intensive and intermediate care unit.
Material and methodsDesignWe conducted a prospective observational study in a level IIIB NICU and NIMCU in a referral hospital between March 2018 and December 2019.
Inclusion criteria- •
Cases of suspected prenatal exposure to drugs of abuse due to the presence of social, health-related and epidemiological risk factors (mothers with past or current substance use, absent or insufficient prenatal care, and/or teenage pregnancy).
- •
Cases of suspected exposure due to clinical manifestations (hypertonia, irritability, inconsolable crying, tremors, strong startle reflex or unexplained intrauterine growth restriction [IUGR]) or the environment of the infant.
- •
Cases in which prenatal exposure was not suspected due to clinical manifestations or environmental factors, but in which mothers provided informed consent for participation in the study.
- •
Meconium aspiration syndrome, intrauterine intestinal perforation, delayed passage of meconium, melena, severe asphyxia or death in the infant.
- •
Neonates without suspected prenatal drug exposure admitted during the weekend or a holiday or from whom a sample of meconium was not obtained.
We collected data on the clinical characteristics of the infants at birth and at discharge and on the diagnostic procedures, management and clinical course during their hospital stay.
We assessed for exposure to drugs of abuse by analysing meconium samples collected within 24 hours of birth. Toxicology testing was performed immediately upon collection, and an aliquot was stored at −20 °C to test for the presence of ethanol at a later time. In the group of patients with suspected exposure, toxicology tests were performed in samples of infant hair and urine if a meconium sample was not available.
Testing of meconium involved a screening test and a confirmation test. We used DRI® immunoassays (Abbott Laboratories Inc., IL, USA) to screen for cannabinoids, cocaine, opiates, methadone, amphetamines and ecstasy.15,16 Positive results were confirmed by gas chromatography–mass spectrometry (GC-MS).15,17,18 The same approach was applied to testing in urine samples.19–21 We tested hair samples directly with GC-MS.19,22,23
We used ethyl glucuronide (ETG) as a marker of prenatal alcohol exposure. We tested meconium samples for the presence of ETG by means of ultra-high performance liquid chromatography with tandem mass spectrometry (UPLC-MS/MS), with limits of detection and quantification of 0.5 ng/g and 5 ng/g, respectively.24 The cut-off to consider the test positive was 30 ng ETG per gram of meconium.25 Testing of hair samples for ETG was performed following a validated method with a limit of quantification of 3 pg/mg, considering results greater than 7 pg/mg positive.26
Statistical analysisWe entered the data for each infant and the results of toxicology tests in a Microsoft Office Excel® 10 spreadsheet. We compared the clinical characteristics, management and outcomes of the newborn patients with negative toxicology results with: a) the group of infants with a positive toxicology test for any drug of abuse, b) the group of infants that tested positive for a single substance and c) the group of infants that tested positive for more than one substance. We also compared the profiles associated with detection of cannabis, alcohol or cocaine in the newborn infant. We performed the same comparative analysis in the subset of infants born after 34 weeks’ gestation.
We have summarised the data as mean ± standard deviation (SD), median and interquartile range (IQR) or absolute frequencies and percentages. We compared groups using unidirectional analysis of variance (ANOVA) and the t test for independent samples or the Kruskal-Wallis and Mann-Whitney U test in case of continuous variables and the χ2 test or Fisher exact test in case of categorical variables. We considered p-values of less than 0.05 (two-tailed) statistically significant.
We used univariate and multivariate binary logistic regression to identify risk factors independently associated with obtaining a positive result in toxicology tests using the group with negative results in all tests as reference (odds ratio [OR] = 1). The initial model included the characteristics of the infant at birth and the reasons for admission to the NICU corresponding to univariate p-values of less than 0.1 (Tables 1–4), except for “substance exposure” as the reason for admission. We built the multivariate model with a stepwise approach, adding variables until completing the model. We established the optimal cut-off points for quantitative variables associated with positive toxicology results by means of receiver operating characteristic (ROC) curves and the Youden index (J), defined as sensitivity + specificity – 1. We used the final binary logistic regression model of the variables independently associated with positive toxicology results to calculate the probability of a positive result in each patient, and based on these probabilities, calculated the area under the ROC curve (AUC) to assess the final model.
Characteristics of newborn infants by prenatal substance exposure group.
| Variable, mean ± SD; n (%); median [interquartile range] | Total sample (N = 372) | Negative toxicology (n = 323) | Positive toxicology (n = 49) | Cannabis (n = 24) | ETG (n = 11) | Cocaine (n = 6) | More than 1 substance (n = 8) |
|---|---|---|---|---|---|---|---|
| Characteristics at birth | |||||||
| Female sex | 166 (44.6%) | 144 (44.6%) | 22 (44.9%) | 10 (41.7%) | 6 (54.5%) | 2 (33.3%) | 4 (50.0%) |
| 1-minute Apgar | 7.2 ± 2.1 | 7.1 ± 2.1 | 7.9 ± 1.8* | 8.4 ± 0.9* | 8.0 ± 1.6 | 7.0 ± 3.5 | 7.3 ± 2.3 |
| 5-minute Apgar | 8.4 ± 1.7 | 8.3 ± 1.7 | 9.1 ± 1.3* | 9.5 ± 0.6* | 8.5 ± 1.8 | 9.4 ± 0.6 | 8.5 ± 2.0 |
| Cord blood pH | 7.28 ± 0.10 | 7.28 ± 0.10 | 7.27 ± 0.07 | 7.28 ± 0.06 | 7.26 ± 0.08 | 7.26 ± 0.10 | 7.28 ± 0.06 |
| Weight (g) | 2266.0 ± 784.5 | 2223.7 ± 789.0 | 2544.6 ± 699.8* | 2454.2 ± 734.1 | 2633.6 ± 656.8 | 2931.7 ± 677.1* | 2403.1 ± 371.7 |
| Length (cm) | 44.7 ± 4.8 | 44.5 ± 4.9 | 46.2 ± 3.8* | 46.0 ± 4.2 | 45.9 ± 2.7b | 49.3 ± 2.8* | 44.9 ± 3.9 |
| HC (cm) | 33.3 ± 3.1 | 31.1 ± 3.0 | 32.6 ± 3.5* | 32.0 ± 2.7 | 32.5 ± 2.0 | 33.2 ± 1.6 | 33.9 ± 7.1 |
| Weight percentile | 36.5 [15.0-63.0] | 38.0 [15.0-63.0] | 26.0 [10.0-60.5] | 18.5 [8.5-52.3]*,a | 42.0 [17.0-83.0] | 45.0 [12.8-79.8] | 20.5 [1.5-32.3] |
| Length percentile | 42.0 [17.0-67.0] | 44.0 [21.0-72.0] | 29.0 [9.0-52.5]* | 25.0 [8.0-49.5]* | 30.0 [12.0-43.0] | 63.0 [33.3-85.3]c | 10.0 [3.0-43.8]* |
| HC percentile | 45.0 [17.0-70.0] | 45.0 [19.0-70.0] | 27.0 [10.5-63.5] | 25.0 [10.3-51.5] | 37.0 [26.0-73.0] | 29.5 [18.8-74.3] | 29.0 [4.5-64.8] |
| GA (weeks) | 35.1 ± 3.7 | 34.8 ± 3.7 | 37.0 ± 3.4* | 36.9 ± 3.4* | 36.5 ± 3.3 | 38.3 ± 2.4* | 37.3 ± 4.2* |
| Degree of prematurity (weeks’ gestation) | |||||||
| < 28 | 11 (3.0%) | 10 (3.1%) | 1 (2.0%) | 1 (4.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 28-34 | 136 (36.6%) | 127 (39.3%) | 9 (18.4%)* | 5 (20.8%) | 2 (18.2%) | 1 (16.7%) | 1 (12.5%) |
| > 34 | 225 (60.5%) | 186 (57.6%) | 39 (79.6%)* | 18 (75.0%) | 9 (81.8%) | 5 (83.3%) | 7 (87.5%) |
| Characteristics at discharge | |||||||
| Weight percentile | 17.0 [6.0-40.0] | 17.5 [6.0-40.0] | 12.0 [4.0-31.5] | 13.5 [4.0-30.0] | 10.0 [8.0-58.0] | 48.0 [6.0-78.3] | 10.5 [1.5-22.3] |
| Length percentile | 29.0 [10.0-55.0] | 29.0 [10.0-56.3] | 23.0 [10.0-53.0] | 18.5 [11.5-51.5] | 23.0 [10.0-55.0] | 52.0 [39.3-70.3] | 15.0 [1.5-34.8] |
| HC percentile | 38.0 [19.0-60.0] | 39.0 [20.0-62.3] | 30.0 [10.0-50.0]* | 26.0 [10.0-55.5] | 38.0 [17.0-50.0] | 28.5 [10.5-53.5] | 18.0 [1.5-46.3]* |
ETG, ethyl glucuronide; GA, gestational age; HC, head circumference; SD, standard deviation.
Characteristics of newborn infants delivered after 34 weeks’ gestation by prenatal substance exposure group.
| Variable, mean ± SD; n (%); median [interquartile range] | Entire group (n = 225) | Negative toxicology (n = 186) | Positive toxicology (n = 39) | Cannabis (n = 18) | ETG (n = 9) | Cocaine (n = 5) | More than 1 substance (n = 7) |
|---|---|---|---|---|---|---|---|
| Characteristics at birth | |||||||
| Female sex | 110 (48.9%) | 93 (50.0%) | 17 (43.6%) | 8 (44.4%) | 5 (55.6%) | 1 (20.0%) | 3 (42.9%) |
| 1-minute Apgar | 7.4 ± 2.1 | 7.3 ± 2.1 | 8.1 ± 1.8* | 8.5 ± 0.9* | 8.4 ± 1.0 | 6.8 ± 4.0 | 7.4 ± 2.4 |
| 5-minute Apgar | 8.7 ± 1.7 | 8.6 ± 1.8 | 9.2 ± 1.3* | 9.6 ± 0.5* | 8.9 ± 1.5 | 9.5 ± 0.6 | 8.6 ± 2.2 |
| Cord blood pH | 7.27 ± 0.10 | 7.26 ± 0.10 | 7.27 ± 0.1 | 7.27 ± 0.07 | 7.27 ± 0.1 | 7.26 ± 0.12 | 7.28 ± 0.1 |
| Weight (g) | 2696 ± 650 | 2683 ± 661 | 2757 ± 597 | 2723 ± 617 | 2809 ± 590 | 3057 ± 675 | 2564 ± 535 |
| Length (cm) | 47.2 ± 3.7 | 47.2 ± 3.8 | 47.4 ± 2.9 | 47.6 ± 3.0 | 46.7 ± 2.2 | 50.1 ± 2.4*,a | 45.9 ± 2.8 |
| HC (cm) | 33.0 ± 2.3 | 32.9 ± 2.0 | 33.5 ± 3.2 | 33.2 ± 1.6 | 33.1 ± 1.6 | 33.4 ± 1.7 | 35.1 ± 6.9 |
| Weight percentile | 29.0 [10.0-56.0] | 30.0 [10.0-57.0] | 17.0 [8.0-42.0] | 11.5 [6.8-36.5] | 40.0 [16.5-72.5] | 23.0 [9.5-80.5] | 15.0 [1.0-30.0]* |
| Length percentile | 38.0 [13.5-62.0] | 40.0 [15.0-63.5] | 17.0 [8.0-46.0]* | 21.0 [7.5-48.5] | 15.0 [11.5-42.0] | 62.0 [25.5-79.5] | 3.0 [3.0-37.0]* |
| HC percentile | 40.0 [14.0-65.0] | 43.5 [15.0-67.3] | 26.0 [10.0-50.0] | 23.0 [10.0-52.5] | 36.0 [25.5-61.5] | 26.0 [13.5-53.5] | 10.0 [3.0-61.0] |
| GA (weeks) | 37.5 ± 2.2 | 37.3 ± 2.2 | 38.5 ± 1.7* | 38.7 ± 1.5* | 37.7 ± 2.0 | 39.2 ± 1.3 | 38.6 ± 2.1 |
| Characteristics at discharge | |||||||
| Weight percentile | 15.5 [4.3-47.0] | 18.0 [5.0-49.0] | 10.0 [3.0-24.0] | 11.5 [3.0-36.5] | 10.0 [5.0-34.5] | 24.0 [5.0-78.5] | 10.0 [1.0-17.0] |
| Length percentile | 30.5 [10.0-61.8] | 32.0 [10.0-63.0] | 19.0 [10.0-54.0] | 18.5 [12.3-52.8] | 16.0 [10.0-44.5] | 54.0 [29.5-78.5] | 10.0 [1.0-36.0] |
| HC percentile | 38.0 [16.0-60.0] | 40.0 [19.0-62.0] | 22.0 [10.0-45.0]* | 18.0 [10.0-43.5] | 32.0 [15.5-47.5] | 24.0 [8.0-48.5] | 3.0 [1.0-50.0]* |
ETG, ethyl glucuronide; GA, gestational age; HC, head circumference; SD, standard deviation.
Diagnosis and management of infants by prenatal substance exposure group.
| Variable, mean ± SD; n (%); median [interquartile range] | Total sample (n = 372) | Negative toxicology (n = 323) | Positive toxicology (n = 49) | Cannabis (n = 24) | ETG (n = 11) | Cocaine (n = 6) | More than 1 substance (n = 8) |
|---|---|---|---|---|---|---|---|
| At admission to NICU | |||||||
| Reason for admission | |||||||
| Preterm birth | 201 (54.0%) | 192 (59.4%) | 9 (18.4%)* | 6 (25%)* | 2 (18.2%)* | 0 (0%)* | 1 (12.5%)* |
| IUGR | 9 (2.4%) | 7 (2.2%) | 2 (4.1%) | 1 (4.2%) | 0 (0%) | 0 (0%) | 1 (12.5%) |
| Asphyxia | 23 (6.2%) | 21 (6.5%) | 2 (4.1%) | 0 (0%) | 1 (9.1%) | 0 (0%) | 1 (12.5%) |
| Low weight | 14 (3.8%) | 10 (3.1%) | 4 (8.2%) | 3 (12.5%) | 1 (9.1%) | 0 (0%) | 0 (0%) |
| Maternal substance use | 20 (5.4%) | 2 (0.6%) | 18 (36.7%)* | 12 (50%)*,a | 0 (0%)b | 4 (66.7%)* | 2 (25%)* |
| Distress | 27 (7.3%) | 26 (8.0%) | 1 (2.0%) | 0 (0%) | 1 (9.1%) | 0 (0%) | 0 (0%) |
| Hypoglycaemia | 17 (4.6%) | 15 (4.6%) | 2 (4.1%) | 0 (0%) | 2 (18.2%) | 0 (0%) | 0 (0%) |
| Seizures | 3 (0.8%) | 3 (0.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Adverse social environment/lack of prenatal care/adoption | 10 (2.7%) | 5 (1.5%) | 5 (10.2%)* | 1 (4.2%) | 1 (9.1%) | 1 (16.7%) | 2 (25.0%)* |
| Heart disease | 6 (1.6%) | 6 (1.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Atresia | 2 (0.5%) | 2 (0.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Malformation, enlarged ventricle | 6 (1.6%) | 6 (1.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hyperbilirubinaemia | 6 (1.6%) | 5 (1.5%) | 1 (2.0%) | 1 (4.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Suspected infection | 5 (1.3%) | 4 (1.2%) | 1 (2.0%) | 0 (0%) | 1 (9.1%) | 0 (0%) | 0 (0%) |
| Anaemia | 2 (0.5%) | 2 (0.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Abdominal distension | 2 (0.5%) | 2 (0.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Other | 19 (5.1%) | 15 (4.6%) | 4 (8.2%) | 0 (0%) | 2 (18.2%) | 1 (16.7%) | 1 (12.5%) |
| Clinical suspicion of prenatal substance exposure | 64 (17.2%) | 28 (8.7%) | 36 (73.5%)* | 20 (83.3%)*,a | 3 (27.3%)b | 6 (100.0%)* | 7 (87.5%)* |
| Multiple pregnancy | 84 (22.6%) | 77 (23.8%) | 7 (14.3%) | 3 (12.5%) | 4 (36.4%) | 0 (0%) | 0 (0%) |
| Diagnosis given during NICU stay | |||||||
| IVH | 54 (14.5%) | 50 (15.5%) | 4 (8.2%) | 2 (8.3%) | 2 (18.6%) | 0 (0%) | 0 (0%) |
| IVH grade I | 44 (11.8%) | 41 (19.5%) | 3 (9.4%) | 2 (15.4%) | 1 (11.1%) | 0 (0%) | 0 (0%) |
| IVH grade II | 8 (2.2%) | 7 (3.3%) | 1 (3.1%) | 0 (0%) | 1 (11.1%) | 0 (0%) | 0 (0%) |
| IVH grade III | 1 (0.3%) | 1 (0.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| IVH grade IV | 1 (0.3%) | 1 (0.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Abstinence syndrome | 4 (1.1%) | 1 (0.3%) | 3 (6.1%)* | 1 (4.2%) | 0 (0%) | 0 (0%) | 2 (25.0%)* |
| Seizures | 7 (1.9%) | 6 (1.9%) | 1 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (12.5%) |
| Leukomalacia | 8 (2.2%) | 7 (2.2%) | 1 (2.0%) | 0 (0%) | 1 (9.1%) | 0 (0%) | 0 (0%) |
| Pneumothorax | 15 (4.0%) | 14 (4.3%) | 1 (2.0%) | 1 (4.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Bronchopulmonary dysplasia | 12 (3.2%) | 12 (3.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Heart disease | 64 (17.2%) | 55 (17.0%) | 9 (18.4%) | 2 (8.3%) | 0 (0%) | 0 (0%) | 1 (12.5%) |
| Patent ductus arteriosus | 30 (8.1%) | 28 (15.2%) | 2 (8.7%) | 0 (0%) | 2 (18.2%) | 0 (0%) | 0 (0%) |
| Necrotising enterocolitis | 5 (1.3%) | 5 (1.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Sepsis | 25 (6.7%) | 24 (7.4%) | 1 (2.0%) | 0 (0%) | 1 (9.1%) | 0 (0%) | 0 (0%) |
| Congenital infection | 2 (0.5%) | 2 (0.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Cytomegalovirus | 3 (0.8%) | 3 (2.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Retinopathy of prematurity | 9 (2.4%) | 8 (3.4%) | 1 (3.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (14.3%) |
| Malformation | 63 (16.9%) | 52 (16.1%) | 11 (22.4%) | 6 (25.0%) | 2 (18.2%) | 0 (0%) | 3 (37.5%) |
| Facial | 12 (3.2%) | 10 (3.1%) | 2 (4.1%) | 1 (4.2%) | 1 (9.1%) | 0 (0%) | 0 (0%) |
| Hepatic | 4 (1.1%) | 3 (0.9%) | 1 (2.0%) | 1 (4.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Gastrointestinal | 6 (1.6%) | 6 (1.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Urogenital | 23 (6.2%) | 19 (5.9%) | 4 (8.2%) | 2 (8.3%) | 0 (0%) | 0 (0%) | 2 (25.0%) |
| CNS | 26 (7.0%) | 21 (6.5%) | 5 (10.2%) | 2 (8.3%) | 2 (18.2%) | 0 (0%) | 1 (12.5%) |
| Management | |||||||
| Surfactant | 35 (9.4%) | 35 (10.8%) | 0 (0%)* | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| NIV | 49 (13.2%) | 46 (14.2%) | 3 (6.1%) | 1 (4.2%) | 1 (9.1%) | 0 (0%) | 1 (12.5%) |
| IMV | 139 (37.4%) | 130 (40.2%) | 9 (18.4%)* | 5 (20.8%) | 2 (18.2%) | 0 (0%) | 2 (25.0%) |
| Home oxygen therapy | 1 (0.3%) | 1 (0.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Inotropic support | 9 (2.4%) | 8 (2.5%) | 1 (2.0%) | 1 (4.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| PN | 128 (34.4%) | 120 (37.2%) | 8 (16.3%)* | 4 (16.7%)* | 2 (18.2%) | 0 (0%) | 2 (25.0%) |
| NGT | 198 (53.2%) | 185 (57.3%) | 13 (26.5%)* | 6 (25.0%)* | 3 (27.3%) | 1 (16.7%) | 3 (37.5%) |
| Chronological age at NGT removal (weeks) | 36.2 ± 1.9 | 36.3 ± 1.9 | 35.6 ± 2.0 | 35.0 ± 1.4 | 36.1 ± 2.0 | 34.4 | 36.8 ± 3.2 |
| BF | 301 (80.9%) | 285 (88.2%) | 16 (32.7%)* | 4 (16.7%)*,a | 9 (81.8%) | 3 (50.0%)* | 0 (0%)* |
| Artificial formula feeding | 278 (74.7%) | 231 (71.5%) | 47 (95.9%)* | 23 (95.8%)* | 10 (90.9%) | 6 (100.0%) | 8 (100.0%) |
| Length of stay (days) | 14.0 [7.0-30.0] | 15.0 [8.0-32.8] | 8 [6.0-15.5]* | 8.5 [5.0-18.5]* | 10.0 [7.0-18.0] | 7.0 [6.5-8.0]* | 9.5 [8.0-23.3] |
BF, breastfeeding; CNS, central nervous system; ETG, ethyl glucuronide; IMV, invasive mechanical ventilation; IVH, intraventricular haemorrhage; IUGR, intrauterine growth restriction; NGT, nasogastric tube; NIV, noninvasive ventilation; PN, parenteral nutrition; SD, standard deviation.
Diagnosis and management of infants delivered after 34 weeks’ gestation by prenatal substance exposure group.
| Variable, mean ± SD; n (%); median [interquartile range] | Entire group (n = 225) | Negative toxicology (n = 186) | Positive toxicology (n = 39) | Cannabis (n = 18) | ETG (n = 9) | Cocaine (n = 5) | More than 1 substance (n = 7) |
|---|---|---|---|---|---|---|---|
| At admission to NICU | |||||||
| Reason for admission | |||||||
| Preterm birth | 55 (24.4%) | 55 (29.6%) | 0 (0%)* | 0 (0%)* | 0 (0%) | 0 (0%) | 0 (0%) |
| IUGR | 9 (4.0%) | 7 (3.8%) | 2 (5.1%) | 1 (5.6%) | 0 (0%) | 0 (0%) | 1 (14.3%) |
| Asphyxia | 23 (10.2%) | 21 (11.3%) | 2 (5.1%) | 0 (0%) | 1 (11.1%) | 0 (0%) | 1 (14.3%) |
| Low weight | 14 (6.2%) | 10 (5.4%) | 4 (10.3%) | 3 (16.7%) | 1 (11.1%) | 0 (0%) | 0 (0%) |
| Maternal substance use | 19 (8.4%) | 2 (1.1%) | 17 (43.6%)* | 12 (66.7%)a | 0 (0%)b | 3 (60%)* | 2 (28.6%)* |
| Distress | 27 (12.0%) | 26 (14.0%) | 1 (2.6%) | 0 (0%) | 1 (11.1%) | 0 (0%) | 0 (0%) |
| Hypoglycaemia | 17 (7.6%) | 15 (8.1%) | 2 (5.1%) | 0 (0%) | 2 (22.2%) | 0 (0%) | 0 (0%) |
| Seizures | 3 (1.3%) | 3 (1.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Adverse social environment/lack of prenatal care/adoption | 10 (4.4%) | 5 (2.7%) | 5 (12.8%)* | 1 (5.6%) | 1 (11.1%) | 1 (20%) | 2 (28.6%)* |
| Heart disease | 6 (2.7%) | 6 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Atresia | 2 (0.9%) | 2 (1.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Malformation, enlarged ventricle | 6 (2.7%) | 6 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hyperbilirubinaemia | 6 (2.7%) | 5 (2.7%) | 1 (2.6%) | 1 (5.6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Suspected infection | 5 (2.2%) | 4 (2.2%) | 1 (2.6%) | 0 (0%) | 1 (11.1%) | 0 (0%) | 0 (0%) |
| Anaemia | 2 (0.9%) | 2 (1.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Abdominal distension | 2 (0.9%) | 2 (1.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Other | 19 (8.4%) | 15 (8.1%) | 4 (10.3%) | 0 (0%) | 2 (22.2%) | 1 (20%) | 1 (14.3%) |
| Clinical suspicion of prenatal substance exposure | 54 (24.0%) | 22 (11.8%) | 32 (82.1%)* | 18 (100%)*,a | 3 (33.3%)b | 5 (100%)* | 6 (85.7%)* |
| Multiple pregnancy | 44 (19.6%) | 39 (21.0%) | 5 (12.8%) | 2 (11.1%) | 3 (33.3%) | 0 (0%) | 0 (0%) |
| Diagnosis given during NICU stay | |||||||
| IVH | 17 (7.6%) | 16 (8.6%) | 1 (2.6%) | 0 (0%) | 1 (11.1%) | 0 (0%) | 0 (0%) |
| IVH grade I | 14 (6.2%) | 13 (12.1%) | 1 (4.2%) | 0 (0%) | 1 (14.3%) | 0 (0%) | 0 (0%) |
| IVH grade II | 2 (0.9%) | 2 (1.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| IVH grade IV | 1 (0.4%) | 1 (0.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Abstinence syndrome | 3 (1.3%) | 1 (0.5%) | 2 (5.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (28.6%)* |
| Seizures | 7 (3.1%) | 6 (3.2%) | 1 (2.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (14.3%) |
| Leukomalacia | 5 (2.2%) | 5 (2.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pneumothorax | 11 (4.9%) | 11 (5.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Heart disease | 27 (12%) | 25 (13.4%) | 2 (5.1%) | 1 (5.6%) | 0 (0%) | 0 (0%) | 1 (14.3%) |
| Patent ductus arteriosus | 9 (4.0%) | 8 (4.3%) | 1 (2.6%) | 0 (0%) | 1 (11.1%) | 0 (0%) | 0 (0%) |
| Sepsis | 10 (4.4%) | 9 (4.8%) | 1 (2.6%) | 0 (0%) | 1 (11.1%) | 0 (0%) | 0 (0%) |
| Malformation | 42 (18.7%) | 34 (18.3%) | 8 (20.5%) | 4 (22.2%) | 1 (11.1%) | 0 (0%) | 3 (42.9%) |
| Facial | 9 (4%) | 8 (4.3%) | 1 (2.6%) | 1 (5.6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Gastrointestinal | 4 (1.8%) | 4 (2.2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Urogenital | 16 (7.1%) | 13 (7.0%) | 3 (7.7%) | 1 (5.6%) | 0 (0%) | 0 (0%) | 2 (28.6%) |
| CNS | 19 (8.4%) | 15 (8.1%) | 4 (10.3%) | 2 (11.1%) | 1 (11.1%) | 0 (0%) | 1 (14.3%) |
| Management | |||||||
| Surfactant | 4 (1.8%) | 4 (2.2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| IMV | 20 (8.9%) | 19 (10.2%) | 1 (2.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (14.3%) |
| NIV | 40 (17.8%) | 39 (21.0%) | 1 (2.6%)* | 0 (0%) | 0 (0%) | 0 (0%) | 1 (14.3%) |
| Home oxygen therapy | 1 (0.4%) | 1 (0.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Inotropic support | 5 (2.2%) | 5 (2.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| PN | 27 (12%) | 25 (13.4%) | 2 (5.1%) | 1 (5.6%) | 0 (0%) | 0 (0%) | 1 (14.3%) |
| NGT | 67 (29.8%) | 63 (33.9%) | 4 (10.3%)* | 1 (5.6%)* | 0 (0%) | 0 (0%) | 2 (28.6%) |
| Chronological age at removal of NGT (weeks) | 37.7 ± 2.1 | 37.7 ± 2.2 | 38.1 ± 1.7 | 37.6 | 38.4 | 0 (0%) | 38.2 ± 2.9 |
| BF | 169 (75.1%) | 156 (83.9%) | 13 (33.3%)* | 0 (0%)*,a | 8 (88.9%) | 3 (60%) | 0 (0%)* |
| Artificial formula feeding | 185 (82.2%) | 148 (79.6%) | 37 (94.9%)* | 17 (94.4%) | 8 (88.9%) | 5 (100%) | 7 (100%) |
| Length of stay (days) | 9.0 [6.0-12.0] | 9.0 [6.0-13.0] | 8.0 [5.0-10.0] | 5.5 [5.0-9.5] | 8.0 [6.5-12.0] | 7.0 [6.0-8.0] | 8.0 [9.0-12.0] |
BF, breastfeeding; CNS, central nervous system; ETG, ethyl glucuronide; IMV, invasive mechanical ventilation; IVH, intraventricular haemorrhage; IUGR, intrauterine growth restriction; NGT, nasogastric tube; NIV, noninvasive ventilation; PN, parenteral nutrition; SD, standard deviation.
The statistical analysis was performed with the software SPSS, version 23.0® (SPSS Inc., Chicago, IL, USA).
ResultsParticipantsWe included 372 (41.6%) of the 895 newborn infants admitted to the NICU and NIMCU in the period under study. The distribution by gestational age was: 225 (60.5%) delivered after 34 weeks, 136 (36.4%) between 28-34 weeks and 11 (3.0%) before 28 weeks. Prenatal exposure to drugs or alcohol was detected in 49 infants (13.2%), of who 39 (79.6%) were born after 34 weeks’ gestation. This was the reason why we performed a separate statistical analysis in this subset of infants.
In the group with positive results, 41 infants (83.7%) tested positive for only 1 substance: 24 (58.5%) for cannabis, 11 (26.8%) for ETG and 6 (14.6%) for cocaine. Eight infants (16.3%) tested positive for more than 1 substance.
Of the 64 infants in the group in which exposure was suspected due to clinical manifestations (17.2%), 36 (56.6%) tested positive, while of the 308 (82.8%) in the group in which exposure was not suspected, 13 (4.2%) tested positive. Urine samples were obtained in 55 infants with suspected exposure (85.9%). The results were positive in 22 urine samples (40%). Hair samples were collected from 12 infants with suspected exposure, and tested positive in 11 cases (91.7%).
Clinical characteristics (Tables 1 and 2)Detected exposure was associated with higher 1- and 5-minutes Apgar scores in infants that tested positive for illicit drugs or cannabis compared to infants with negative results. These results were maintained in the group delivered after 34 weeks’ gestation (Tables 1 and 2).
The median birth length percentile of infants exposed to any substance (29.0; IQR, 9.0-52.5), more than 1 substance (10.0; IQR, 3.0-43.8) or cannabis in particular (25.0, IQR, 8.0-49.5) was lower compared to infants without exposure (44.0; IQR, 21.0-72.0) (P < .05). The birth length percentile was also lower in infants exposed to cannabis compared to those exposed to cocaine (25.0 [IQR, 8.0-49.5] vs 63.0 [IQR, 33.3-85.3]; P < .05). The median weight percentile of infants exposed to cannabis (18.5; IQR, 8.5-52.3) was lower compared to infants without exposure (38.0; IQR, 15.0-63.0) (P < .05) and infants exposed to alcohol (42.0; IQR, 17.0-83.0) (P < .05).
In the subset of infants born after 34 weeks’ gestation, the median birth length percentile of infants exposed to any substance (17.0; IQR, 8.0-46.0) or more than 1 substance (3.0; IQR, 3.0-37.0) was lower compared to infants without exposure (40.0; IQR, 15.0-63.5) (P < .05). The median birth weight percentile of infants exposed to more than 1 substance (15.0; IQR, 1.0-30.0) was also lower compared to infants without exposure (30.0; IQR, 10.0-57.0) (P < .05).
At discharge, the head circumference of infants exposed to any substance (22.0; IQR, 10.0-45.0) and more than 1 substance (3.0; IQR, 1.0-50.0) was lower compared to infants without exposure (40.0; IQR, 19.0-62.0) (P < .05). These differences were also observed in the group delivered after 34 weeks’ gestation.
The mean gestational age at birth of infants exposed to any substance (37.0 ± 3.4 weeks), more than 1 substance (37.3 ± 4.2 weeks), to cannabis (36.9 ± 3.4 weeks) and to cocaine (38.3 ± 2.4) was higher compared to infants without exposure (34.8 ± 3.7 weeks) (P < .05). In the subset born after 34 weeks, gestational age was higher in the groups exposed to any substance (38.5 ± 1.7 weeks) or to cannabis (38.7 ± 1.5 weeks) compared to the group without exposure (37.3 ± 2.2. weeks) (P < .005).
We did not find significant differences in any other variable.
Diagnosis and clinical managementAdmission due to preterm birth was more frequent in infants without detected exposure compared to infants with detected exposure (59.4% vs 18.4%; P < .05) (Tables 3 and 4).
The most frequent reason for admission in infants with detected exposure was known maternal substance use (36.7%; specifically, cannabis [50%], cocaine [66.7%] and more than 1 substance [25.0%]), with greater frequency compared to the group of infants without exposure (0.6%) (P < .05). We also observed these differences in the subset of infants born after 34 weeks’ gestation.
Admissions due to adverse social/environmental conditions, absent prenatal care or adoption were more frequent in infants with detected exposure to more than 1 substance compared to infants with negative toxicology results (25% vs 1.5%; P < .05).
Suspicion of exposure due to clinical manifestations was more frequent in infants that tested positive for any substance (73.5%), cannabis (83.3%), cocaine (100%) or more than 1 substance (87.5%), compared to infants with negative results (17.2%) (P < .05). These differences were also significant in the group born after 34 weeks’ gestation.
When it came to the diagnoses, we found a higher frequency of diagnosis of neonatal abstinence syndrome in infants with positive toxicology results compared to infants with negative results (6.1% vs 0.3%; P < .05). It was also more frequent in infants positive for more than 1 substance compared to infants with negative results in both the overall sample (25.0% vs 0.3%; P < .05) and in the subset born after 34 weeks’ gestation (28.6% vs 0.5%; P < .05). The single case diagnosed in an infant with negative results corresponded to the child of a mother receiving methadone maintenance treatment.
As for clinical management, infants with positive toxicology results were associated with a decreased need of surfactant (10.8% vs 0%; P < .05) and noninvasive ventilation (NIV) (40.2% vs 18.4%; P < .05) compared to infants with negative results admitted to the NICU and NIMCU. In the group delivered after 34 weeks, we found a decreased frequency of NIV (21% vs 2.6%; P < .05) and of nasogastric tube (NGT) placement (33.9% vs 10.3%; P < .05) in the exposed vs not exposed group, with less frequent NGT placement in the group of infants exposed to cannabis. Parenteral nutrition (PN) was less frequent in the group exposed to any substance (16.3%) and the group exposed to cannabis (16.7%) compared to the group without exposure (37.2%) (P < .05),
In the group of exposed infants compared to those without exposure, the prevalence of breastfeeding (BF) was lower (32.7% vs 88.2%; P < .05) and the use of artificial formula (AF) greater (95.9% vs 71.5%; P < .05) except in infants exposed to alcohol (BF: 81.8%; AF: 90.9%), who were not identified during the hospital stay. These differences were also significant in the group born after 34 weeks’ gestation.
Lastly, the median length of stay was shorter in infants exposed to any drug of abuse (8.0 days; IQR, 6.0-15.5), cannabis (8.5 days; IQR, 5.0-18.5) or cocaine (7.0; IQR, 6.5-8.0) compared to infants without exposure to substances (9.5; IQR, 8.0-23.3) (P < .05). These differences were also significant in the group born after 34 weeks’ gestation.
We ought to note that although the difference was not statistically significant (0.05 < P < 0.1), genitourinary malformations were more frequent in infants exposed to more than one substance compared to infants with negative toxicology results (25% vs 5.9%; P = .085), which was also the case in the subset delivered after 34 weeks’ gestation (28.6 vs 7.0%; P = .094).
We did not find significant differences in any other variables.
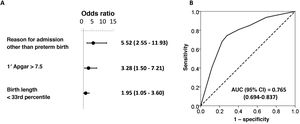
Risk factors independently associated with positive toxicology resultsIn the final model for the overall sample of hospitalised neonates, the risk factors independently associated with positive toxicology results were: reason for admission other than prematurity (OR, 5.52; 95% confidence interval [CI], 2.55-1.93), 1-minute Apgar greater than 7.5 (OR, 3.28; 95% CI, 1.50-7.21) and birth length below the 33rd percentile (OR, 1.95; 95% CI, 1.05-3.60) (Fig. 1). In the group delivered after 34 weeks, the independent risk factors were: admission due to adverse social/environmental conditions or lack of prenatal care (OR, 4.47; 95% CI, 1.03-19.29), 1-minute Apgar score greater than 7.5 (OR, 3.38; 95% CI, 1.33-8.54) and birth length below the 33rd percentile (OR, 2.14; 95% CI, 1.04-3.40) (Fig. 2).
Binary logistic regression analysis of risk factors associated with positive toxicology test results in the total sample of neonates admitted to the NICU and NIMCU. Forest plot (A) and ROC curve (B) of the obtained multivariate model. The table presents the crude and adjusted odds ratios (ORs) with the corresponding confidence intervals in parentheses. The optimal cut-off values were established by the maximum value of the Youden index (J), defined as sensitivity + specificity – 1. We compared expected and observed frequencies using the Hosmer-Lemeshow goodness of fit test (P = .969) and comparing ROC curves and the areas under the curve (AUCs), which confirmed that the model was a good fit.
Binary logistic regression analysis of risk factors associated with positive toxicology test results in the group of infants delivered after 34 weeks’ gestation admitted to the NICU and NIMCU. Forest plot (A) and ROC curve (B) of the obtained multivariate model. The table presents the crude and adjusted odds ratios (ORs) with the corresponding confidence intervals in parentheses. The optimal cut-off values were established by the maximum value of the Youden index (J), defined as sensitivity + specificity – 1. We compared expected and observed frequencies using the Hosmer-Lemeshow goodness of fit test (P = .969) and comparing ROC curves and the areas under the curve (AUCs), which confirmed that the model was a good fit.
The clinical characteristics and risk factors identified in our study could help guide the early identification of infants exposed to substances. This is particularly relevant for hospitals that do not have the means to detect exposure to these drugs and to guide ordering of tests for markers of exposure in biological matrices where available.
To our knowledge, this is the first study of these characteristics conducted in Spain. The comparison of the effects associated with different drugs of abuse is a novel perspective and allowed us to minimise the biases that result from comparing outcomes in different populations.
Clinical characteristicsThe impact on infants of prenatal drug exposure depends on multiple factors (type of substance, timing of exposure, duration of exposure, route of administration, nutritional status, maternal physical and mental health and quality of prenatal care).6,7
The analysis of anthropometric parameters revealed that neonates with prenatal substance exposure had values at lower percentiles, with marked differences in the group exposed to cannabis. Although other studies have found an association between lower anthropometric values and exposure to cannabis,27,28 there are also studies in the literature that did not find evidence of it.4,13,29
It is known that infants with a history of IUGR are at greater risk complications in the perinatal and immediate postnatal periods. It is also important to monitor development, mainly in the first years of life, in order to diagnose and treat developmental delays early. It is known that exposure to unfavourable environmental conditions in a short but critical period can disturb the interaction of endocrine systems and metabolic and haemodynamic homeostasis mechanisms. The problem posed by IUGR is that it is difficult to reach a certain diagnosis.30
The results of our study could help expand the differential diagnosis and identification of the possible causes of anthropometric abnormalities in newborn infants by considering exposure to drugs of abuse.
In addition, they could be used to guide the development of preventive strategies and policies for prevention and detection of prenatal substance exposure.31,32
Diagnosis and clinical managementAs would be expected, the reasons for admission associated with suspected prenatal substance exposure were more frequent in exposed infants, with the exception of infants exposed to alcohol, which was consistent with the fact that alcohol is the substance identified most frequently in cases in which there is no suspicion.
In 3 out of 4 infants with a positive toxicology test result, there had been clinical suspicion of exposure, while exposure had not been suspected in 1 of 4 cases. Thus, clinical suspicion is a strong predictor of detected exposure. However, further research is required to corroborate these findinds.
Previous studies have analysed the agreement between maternal self-report of substance use and confirmation by toxicology testing.4,11,13,31
Cases of neonatal abstinence syndrome (NAS) have increased with the global increase in the prevalence of opioid dependence observed in the last decade.1,3 Although the incidence of NAS in Spain has not reached the magnitude observed in the United States, where cases increased fivefold in a single decade,1 the 2 infants in our study that tested positive for opiate exposure received a diagnosis of NAS. Symptoms similar to those of NAS have been observed in neonates exposed to cannabis or cocaine.6,27,33 As was the case in our study, there is no evidence of an association between prenatal ethanol exposure and NAS.6
The current evidence on the teratogenicity of cannabis, cocaine or opiates is inconclusive.6,27 Our findings suggest a potential association between prenatal exposure to more than 1 substance and the presence of genitourinary anomalies, which had not been described in the reviewed literature.
Foetal alcohol syndrome and FASD may manifest with facial and central nervous system (CNS) malformations.8 We found CNS malformations in 2 infants exposed to alcohol, one of who also had facial malformations. Exposure was not suspected in any case, and a positive result for ETG allowed the diagnosis of FAS in 1 case. A case of FAS had been confirmed previously in our NICU.5
When it came to management, due to the risks associated with prenatal substance exposure, infants with suspected exposure were admitted for closer monitoring. This is the reason why the group with detected exposure required fewer supportive measures (surfactant, NIV, parenteral nutrition, NGT placement) compared to the group without exposure in a tertiary care neonatal unit, and their higher gestational age was probably also a contributing factor. Patients with prenatal substance exposure were also less likely to have the medical complications associated with other reasons for admission to the NICU or NIMCU.
Breastfeeding is contraindicated in case of maternal substance use6,27 at least until maternal toxicology test results become negative. Consequently, the frequency of BF was low in the group of infants with known substance exposure.
Risk factors independently associated with positive toxicology resultsFrom a clinical standpoint, a reason for admission other than preterm birth, a birth length below the 33rd percentile or adverse social or environmental conditions/poor or absent prenatal care in infants born after 34 weeks’ gestation are useful predictors for identification of prenatal exposure to drugs of abuse and to support existing clinical suspicion.
The fact that a 1-minute Apgar score greater than 7.5 emerged as an independent predictor of exposure could be explained by the greater severity of disease and greater degree of prematurity of the infants without prenatal substance exposure admitted to the NICU and NIMCU.
Toxicology resultsIn our study, the use of different neonatal biological matrices was essential to confirm exposure to drugs of abuse in suspected cases. Traditionally, screening for drug exposure during gestation was performed in urine samples.3 However, in the group of infants with suspected exposure in our sample, a urine sample could not be collected in 9 cases (14.1%), and in 11 (33.3%) of the cases that had positive toxicology results in which a urine sample had been collected, testing of urine yielded negative results. Hair was analysed in 4 (6.3%) infants for whom a meconium sample was not available or was too small for analysis, and in 8 infants (12.5%) to confirm the results of screening.
There are limitations to our study. 1) The impact of foetal alcohol exposure was not known at the time of hospitalization, which prevented clinical decision-making for immediate intervention at the time. 2) We did not include healthy infants as a control group, which may have been a source of bias in our analysis of variables such as the Apgar score, length of stay or the need of NIV. However, the difficulty in obtaining a large enough sample of such participants is well known. 3) We did not include markers of tobacco exposure in the analysis. 4) Testing of meconium can only detect substance exposure in the second and third trimesters of pregnancy.
In conclusion, there are anthropometric and risk factors associated with prenatal exposure to drugs of abuse that may assist in the early identification of neonates with such a history or reinforce an already existing suspicion. In addition, anthropometric abnormalities can help expand the differential diagnosis of detected alterations and guide the investigation of their aetiology. The detection of substance exposure during the care episode allowed for more specific clinical management, discontinuation of BF in case of continued maternal substance use and early initiation of social welfare services and follow-up of the family.
FundingThe study was funded through project 2017I033 (Estudio de la incidencia de la exposición prenatal a alcohol y otras sustancias de abuso en recién nacidos ingresados en una sección de neonatología mediante la determinación de biomarcadores en matrices biológicas) of the National Plan on Drugs of the Ministry of Health, Social Services and Equality of Spain.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all mothers and partners that agreed to the participation of their babies in the study. We all so thank the entire staff of the neonatal unit (physicians, nurses, nursing aides and social workers) and the staff of the Toxicology Laboratory of the Department of Clinical Analysis of the Hospital Universitario Son Espases.
Please cite this article as: Roca A, Jarque P, Gomila I, Marchei E, Tittarelli R, Elorza MÁ, et al. Características clínicas y factores de riesgo asociados a la exposición prenatal a drogas de abuso. An Pediatr (Barc). 2021;95:307–320.