Necrotizing pneumonia (NP) is a serious complication of community-acquired pneumonia characterised by the destruction of normal lung parenchyma. No study has evaluated the repercussions of the lung damage in the years following the episode. The aim of this study was to assess the long-term impact on lung function and respiratory symptoms in children hospitalised due to NP.
MethodsWe analysed outcomes in children given a diagnosis of NP between January 2003 and April 2016. We selected patients aged more than 4 years capable of undergoing a lung function test, that had been followed up for at least 2 years. The patients completed a respiratory questionnaire and underwent a lung function test.
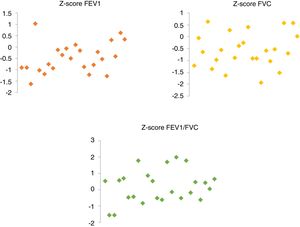
ResultsWe included a total of 24 patients (12 male). The median age at the time of diagnosis was 28 months, the median length of stay was 15 days, and 18 patients required pleural drainage. The mean duration of follow-up after NP was 8.75 years. During the evaluation, none of the patients exhibited asthma, cough, or exercise-induced symptoms. Three children had a second episode of pneumonia that did not require hospital admission. The spirometry results were the following (given as mean±standard deviation): FEV1 Z-score, −0.47±0.65; FVC Z-score, −0.56±0.73; and FEV1/FVC Z-score, 0.19±0.98. We found no evidence of obstructive pulmonary disease or restrictive patterns.
ConclusionsThe long-term outcomes of paediatric NP are good. However, patients exhibited mildly impaired lung function several years after the episode. We recommend follow-up of these patients due to potential impairments in lung function in adulthood.
La neumonía necrotizante (NN) es una complicación grave de la neumonía adquirida en la comunidad caracterizada por la destrucción del parénquima pulmonar normal. Ningún estudio ha evaluado las consecuencias de este daño pulmonar en los años posteriores al episodio. El objetivo es investigar el impacto a largo plazo sobre la función pulmonar y los síntomas respiratorios en niños ingresados por NN.
MétodosSeguimiento de niños diagnosticados de NN desde enero-2003 hasta abril-2016. Se seleccionaron a los mayores de 4 años, capaces de realizar una función pulmonar, y un seguimiento durante más de 2 años. Los pacientes recibieron un cuestionario respiratorio y completaron una prueba de función pulmonar.
ResultadosSe incluyeron 24 pacientes (12 hombres). La edad mediana en el momento del diagnóstico fue de 26 meses, 15 días de hospitalización y 18 pacientes necesitaron drenaje pleural. Los pacientes fueron seguidos durante un promedio de 8.75 años después de la NN. Durante la evaluación, ningún paciente tuvo asma, tos o sintomatología inducida por el ejercicio. Tres niños sufrieron una segunda neumonía, que no requirió hospitalización. Los resultados de la espirometría fueron (media±desviación estándar): Z-score FEV1 −0.47±0.65, Z-score FVC −0.56±0.73, Z-score FEV1/FVC 0.19±0.98. No hubo evidencia de enfermedad pulmonar obstructiva o patrones restrictivos.
ConclusionesLos resultados a largo plazo de la NN pediátrica son buenos. Sin embargo, los pacientes tienen una función pulmonar ligeramente disminuida varios años después del episodio. Es aconsejable hacer un seguimiento de estos pacientes debido a la posible disminución de la función pulmonar en edad adulta.
Necrotizing pneumonia (NP) is an uncommon but severe complication of bacterial pneumonia that carries a high morbidity and may even result in death. Pathology reports from autopsies or examination of surgical lung specimens describe pulmonary inflammation, alveolar consolidation and thrombosis of intrapulmonary vessels, with accompanying necrosis and multiple small cavities. It has been hypothesised that the reduction in blood flow from thrombosed vessels results in decreased antibiotic concentrations in the affected lung tissue, which in turn results in persistent infection and further destruction of pulmonary tissue.1–41–3 Although the presentation of NP varies widely, most children experience severe disease with high and prolonged fever, dyspnoea, clinical and radiological signs of extensive lung consolidation and prolonged hospital stays. It is often associated with pleural effusion, empyema, pyopneumothorax and bronchopleural fistulae.4–83–7
Several recent studies have described the association of a history of pneumonia during childhood with long-term pulmonary sequelae and impaired lung function in adulthood.8–10
Given that pneumonia can have a negative impact on lung function outcomes, it is reasonable to assume that necrotizing pneumonia may cause even greater lung damage. However, there are no studies in the literature assessing the long-term impact of NP in the lungs of children.
The aim of our study was to investigate the long-term pulmonary function test results and respiratory symptoms in children with a history of NP.
Patients and methodsPatientsThe study population consisted of patients aged 0–14 years admitted with a diagnosis of necrotizing pneumonia to the Hospital Universitari Son Espases in Palma de Mallorca, Spain, between January 2003 and April 2016. This is the only tertiary care hospital in the Balearic Islands. It serves a population of about 1 100 000 inhabitants, of who 180 000 are children aged less than 15 years. The diagnosis of NP was based on the presence on chest imaging of solitary or multiple thin-walled cavities within areas of consolidation, excluding abscesses.11,12
For each patient, we collected data on demographic characteristics, laboratory test results, microbiological cultures and clinical information obtained at admission, during the hospital stay and in any subsequent outpatient follow-up visit to the hospital. Microbiological tests included pharyngeal swab or sputum cultures, blood cultures and pneumococcal antigen tests and culture of pleural effusion samples (in case of pleural drainage).
We included children followed up for at least 2 years after the episode of NP who were able to collaborate in spirometry for pulmonary function testing.
We excluded patients that had chronic respiratory diseases before the NP episode or with current disease preventing them from performing appropriately in spirometry. A total of 35 patients were eligible for the study, but we were unable to reach 5 of them and 6 lived outside the island, so the final sample consisted of 24 patients.
The study was approved by the Ethics Committee of the Hospital Universitari Son Espases (IB 3655/18 PI). Before enrolling patients, we obtained the signed informed consent of the patient and parents for patients aged less than 18 years.
Respiratory questionnaire and physical examinationAt the time of the evaluation, in addition to information on respiratory manifestations, we collected data on the following: personal history, family history of atopy, parental smoking, history of pneumonia or bronchitis before or after NP and use of bronchodilator treatment. We administered a standardized respiratory questionnaire to rule out asthma (International Study of Asthma and Allergies in Childhood [ISAAC] questionnaire) and performed a complete physical examination in each patient.13
Pulmonary function testingWe performed pulmonary function tests with a Jaeger Masterlab spirometer (Jaeger AG, Würzburg, Germany). We determined the standard values using the equations of the Global Lung Function Initiative (GLI) of 2012, with the new reference values for age, sex, weight, race and height registered and validated by the European Respiratory Society (ERS) in 2012.14–17 The equations of the GLI, which have been endorsed by the major respiratory societies, approximate the ideal, since the data provide a smooth representation of the 3–95 years age range and cover five ethnic groups. Quanjer et al. obtained predicted values using the GLI-2012 equations taking into account data variation and skewness to calculate the lower limit of normal (LLN) and z-score distribution.14 We calculated the LLNs, defined as the 5th percentile (z=–1.64), by applying the pertinent prediction equations. We used the z-score distribution, as recommended by the American Thoracic Society (ATS) and the ERS for lung function testing.18,19
At the time of testing, patients had to be asymptomatic and free of any intercurrent conditions that could influence the result. The main variables measured in the test were the forced vital capacity (FVC), the forced expiratory volume in the first second (FEV1) and the FEV1/FVC ratio.
We defined airway obstruction as a FEV1/FVC below the LLN and a restrictive pattern in spirometry as a FEV1/FVC at or above the LLN with a FVC below the LLN.
All pulmonary function tests had to meet the criteria established by the ATS/ERS to be considered valid.19,20
Statistical analysisWe have expressed the data as mean±standard deviation (SD) and/or percentile, median and interquartile range (IQR) or absolute frequency and percentage distributions. We compared groups using the Mann-Whitney U tests in case of continuous variables and the chi-square test in case of categorical variables. We defined statistical significance as 2-tailed p-value of less than 0.05. The statistical analyses were performed with the software SPSS 23.0 (SPSS Inc., Chicago, IL, USA).
ResultsNecrotizing pneumonia was diagnosed in 35 patients between January 2003 and April 2016. Of the 35 patients eligible for the study, 24 enrolled (12 female, 12 male). There were 8 patients for the 2000–2007 period (with no cases for 2000–2002) and 16 patients for the 2008–2016 period. The median age at the time of diagnosis was 26 months (IQR, 17.25−47 months) and the median length of stay was 15 days (IQR, 12.25−18 days). During the hospital stay, the patients had fever for a median of over 16 days (IQR, 10.5−17 days). The most frequently detected pathogen was Streptococcus pneumoniae, found in 13 patients (in 1 case in coinfection with Chlamydophila pneumoniae) and adenovirus was the causative agent in 1 patient; in the remaining 10 patients, the microbiological test results were negative. Twenty-one patients had pleural effusion, meeting the criteria for empyema in 17. All patients received intravenous antibiotics. The median duration of intravenous antibiotherapy was 14 days (IQR, 10.75−15.25 days) and the median of intravenous and oral treatment was 21 days (IQR, 19.75–23.25 days). In addition to antibiotherapy, 18 patients needed pleural drainage and 11 fibrinolytics, and none of the patients required surgery. Air leaks (pneumothorax and/or bronchopulmonary fistula) were detected in 10 patients with NP. The pneumothorax and bronchopulmonary fistulae resolved spontaneously in all, and none required surgical management. There were no deaths in the sample (Table 1).
Characteristics of patients with pneumonia.
| Age at admission (months) | Sex (male/female) | Duration of fever before admission (days) | Aetiology | Microbiological diagnosis | White blood cells | Neutrophils | CRP (mg/dL) | Total duration of fever (days) | Antibiotic treatment (inpatient) | Duration of inpatient antibiotic treatment (days) | Pleural drainage (0=No, 1=Yes) | Fibrinolytic treatment (0=No, 1=Yes) | Complications | Length of stay (days) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 47 | F | 4 | SP | Pneumococcal Ag in PF | 19490 | 12 300 | 11.4 | 16 | cefotaxime | 12 | 1 | 1 | PNT | 16 | |
| 30 | F | 3 | SP | Pneumococcal Ag in PF | 28360 | 22 600 | 29.5 | 15 | cefotaxime | 14 | 1 | 1 | PNT | 18 | |
| 23 | M | 5 | SP | Blood culture | 18160 | 8950 | 44.3 | 13 | cefotaxime | 10 | 0 | 0 | 11 | ||
| 15 | F | 7 | SP | Pneumococcal Ag in PF and PF culture | 31550 | 27 780 | 28 | 10 | cefotaxime | 17 | 1 | 1 | PNT | 13 | |
| 39 | F | 5 | SP | PF culture | 28460 | 19 922 | 29.6 | 8 | cefotaxime | 14 | 1 | 1 | PNT | 15 | |
| 17 | M | 2 | -- | 19790 | 8905.5 | 12.1 | 12 | cefotaxime+vancomycin | 12 | 1 | 0 | Fis+PNT+PNM | 20 | ||
| 13 | M | 6 | -- | 14550 | 9894 | 43 | 20 | cefotaxime | 13 | 1 | 1 | PE | 15 | ||
| 72 | F | 3 | SP+CP | PF culture and serology | 10300 | 8188.5 | 28.6 | 15 | cefotaxime+vancomycin+clarithromycin | 15 | 1 | 0 | Emp | 15 | |
| 34 | F | 5 | SP | Pneumococcal Ag in PF | 28100 | 22 480 | 12 | 15 | cefotaxime+vancomycin | 21 | 1 | 1 | Emp+PNT | 34 | |
| 157 | M | 6 | – | 23740 | 21 130 | 41.7 | 18 | cefotaxime+vancomycin | 23 | 0 | 0 | 23 | |||
| 29 | M | 5 | – | 20440 | 10 220 | 23.9 | 17 | cefotaxime | 14 | 0 | 0 | 15 | |||
| 64 | M | 4 | – | 21050 | 15 156 | 52.4 | 12 | cefotaxime | 14 | 0 | 0 | Emp | 15 | ||
| 53 | F | 7 | SP | Pneumococcal Ag in PF | 12800 | 8740 | 20.6 | 8 | cefotaxime | 10 | 1 | 1 | Emp | 12 | |
| 19 | F | 4 | – | 14810 | 9256 | 10 | cefotaxime | 14 | 1 | 0 | Emp | 15 | |||
| 11 | M | 4 | A | Pharyngeal swab | 18000 | 15 480 | 33.5 | 15 | cefotaxime+vancomycin | 14 | 1 | 0 | PE | 16 | |
| 18 | F | 7 | – | 19600 | 5.8 | 15 | cefotaxime+vancomycin | 1 | 1 | Emp | 21 | ||||
| 28 | F | 15 | – | 26320 | 18 200 | 17.6 | 23 | cefotaxime+cloxacillin | 10 | 0 | 0 | PE | 11 | ||
| 19 | M | 10 | – | 17100 | 5300 | 6.4 | 10 | cefotaxime | 8 | 1 | 1 | Emp | 8 | ||
| 12 | M | 7 | SP | Pneumococcal Ag in PF | 40300 | 24 600 | 32 | 7 | cefotaxime | 15 | 1 | 1 | Emp+PNT+Fis | 18 | |
| 54 | F | 5 | SP | Pneumococcal Ag in PF | 15300 | 9870 | 15.5 | 12 | cefotaxime | 11 | 1 | 1 | PE | 11 | |
| 47 | M | 6 | – | 22900 | 15 570 | 21.5 | 16 | cefotaxime | 9 | 0 | 0 | PE | 11 | ||
| 24 | M | 9 | SP | Pneumococcal Ag in PF and PF culture | 32000 | 22 470 | 18.1 | 20 | Cefotaxime+erythromycin | 16 | 1 | 0 | Emp | 18 | |
| 14 | M | 6 | SP | Pneumococcal Ag in PF | 10800 | 27 | 26 | Multiple changes | 1 | 0 | PNT+Fis | 48 | |||
| 24 | F | 3 | SP | Blood culture | 7330 | 6110 | 29 | 17 | cefotaxime+vancomycin | 16 | 1 | 0 | Emp | 18 | |
| Median | 26 | 5 | 19 695 | 13 728 | 25.5 | 15 | 14 | 15 | |||||||
| IQR | 1725−47 | 4−7 | 14 932.5−27 655 | 8938.9–21 465 | 16−31.4 | 10.5−17 | 10.75−15.25 | 12.25−18 |
A, adenovirus; Ag, antigen; CP, Chlamydophila pneumoniae; CPR, C-reactive protein; Emp; empyema; Fis, fistula; IQR, interquartile range; PF, pleural fluid; PNM, pneumomediastinum; PNT, pneumothorax; SP, Streptococcus pneumoniae.
At the time of evaluation, the median age of the patients was 10.9 years (IQR, 8.3–14.2 years). The median duration of follow-up (from the episode of NP to the last evaluation including a pulmonary function test) was 8.75 years (IQR, 6–10.8 years). At the time of the visit, the findings of the physical examination were normal in all patients, without abnormal or asymmetrical breathing sounds. Ten children had a family history of atopy (41.7%), five had parents that smoked (20.8%), and none reported a history of pneumonia before the episode of NP. Four patients had had wheezing before the episode of NP and 3 after the episode, with no asthma-related respiratory symptoms in the past year. None of the patients reported coughing or symptoms associated with exercise. During the follow-up, 3 children had a second episode of pneumonia that had no complications and did not require hospital admission. Patients completed the standardized respiratory questionnaire (ISAAC) at the time the spirometry test was performed. The ISAAC questionnaire results revealed absence of wheezing in the last 12 months in 22 patients. The other 2 patients reported isolated episodes of wheezing in the previous year. One patient had wheezing in early childhood (successfully treated) but did not have any more episodes in recent years.
Spirometry was performed in all patients during the study visit. Applying the GLI reference equations for spirometry, we did not detect an obstructive pattern in any of the patients. The FEV1 and FEV1/FVC ratio were above the LLN in all cases: the mean FEV1 z-score was –0.47±0.65 (34th percentile), the FEV1/FVC z-score was 0.19±0.98 (54.8th percentile). Only 1 patient had a FVC z-score below the LLN (z, –1.94; 3rd percentile). The mean FVC z-score was –0.56±0.73 (32.8th percentile) (Fig. 1).
We found no statistically significant differences in pulmonary function based on the need of pleural drainage or the presence of an air leak (Tables 2 and 3). All patients in both groups had a normal FEV1 (> LLN). We did not find statistically significant differences in the FVC between patients that required drainage (94.4% with a normal FVC) and those that did not need it (100% with a normal FVC) (P=.55). All patients with an air leak had a normal FVC compared to 93.7% of the patients without an air leak, but this difference was not statistically significant (P=.47).
Pulmonary function test in patients that required pleural drainage versus patients that did not. Groups compared with the Mann-Whitney U test.
| PLEURAL DRAINAGE | NO PLEURAL DRAINAGE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentile | Percentile | ||||||||||||
| n | Mean | Standard Deviation | 25th | 50th (Median) | 75th | n | Mean | Standard Deviation | 25th | 50th (Median) | 75th | P | |
| FEV1 z | 18 | –0.4667 | 0.66779 | –0.9475 | –0.6350 | –0.0700 | 6 | –0.4950 | 0.65948 | –0.9850 | –0.4200 | 0.0275 | .790 |
| FVC z | 18 | –0.629 | 0.7861 | –1238 | –0.805 | 0.080 | 6 | –0.365 | 0.5151 | –0.715 | –0.490 | –0.048 | .301 |
| FEV1/FVC z | 18 | 0.3578 | 0.98180 | –0.4300 | 0.4500 | 0.9300 | 6 | –0.2933 | 0.86920 | –0.8225 | –0.5100 | 0.6025 | .172 |
FEV1, forced expiratory volume in the first second; FVC, forced vital capacity.
Pulmonary function test in patients with pneumothorax or bronchopulmonary fistula versus patients without. Groups compared with the Mann–Whitney U test.
| PNEUMOTHORAX OR BPF | NO PNEUMOTHORAX OR BPF | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Standard Deviation | Percentile | n | Mean | Standard Deviation | Percentile | ||||||
| 25th | 50th (Median) | 75th | 25th | 50th (Median) | 75th | P | |||||||
| FEV1 z | 8 | –0.3800 | 0.82505 | –1.0000 | –0.7250 | 0.4400 | 16 | –0.5206 | 0.57089 | –0.9075 | –0.4600 | –0.0925 | .951 |
| FVC z | 8 | –0.323 | 0.7995 | –1.140 | –0.290 | 0.515 | 16 | –0.684 | 0.6820 | –0.990 | –0.685 | –0.293 | .342 |
| FEV1/FVC z | 8 | –0.1450 | 0.76381 | –0.7150 | –0.0600 | 0.5475 | 16 | 0.3650 | 1.05201 | –0.5150 | 0.4500 | 1.4800 | .408 |
BPF, bronchopulmonary fistula; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity.
Necrotizing pneumonia (NP) is a severe complication of community-acquired pneumonia characterised by liquefaction and cavitation of the lung parenchyma. Nearly 4% of all community-acquired pneumonias are necrotizing, although retrospective studies analysing the incidence of NP have found an increasing trend in the past few decades.1–4,7 Current knowledge on NP is based mainly on case reports and small case series.21 There are few publications with data from large case series.22,23 A French study provided a better estimate on the local incidence of necrotizing pneumonia: between May 2006 and April 2011, there were 41 cases of NP (0.8% of total cases of pneumonia), but the percentage doubled when the authors compared the 2006–2009 period to the 2009–2011 period.23
In our study, we found 35 paediatric cases of NP diagnosed over a 13-year period in our hospital. There was significant radiographic evidence of lung damage. Necrotizing pneumonia was detected in children with increasing frequency over time. The most frequently identified causative agent was S. pneumoniae, which was consistent with other studies.24–26 We found no cases of M. pneumoniae or S. aureus infection, contrary to other case series in the literature.27,28 Most patients with NP also develop complications such as parapneumonic effusion, empyema or bronchopleural fistulae.29 The duration of symptoms is longer in NP despite early initiation of therapy, as is the length of stay.30 The treatment of NP is usually prolonged, requiring a prolonged course of intravenous antibiotics and possibly insertion of a pleural drainage tube.1–4,7 In our study, 75% of the patients required pleural drainage tube and were hospitalised for 15 days. Nevertheless, the prognosis of NP is usually good, and most respiratory symptoms and radiological abnormalities resolve in a few weeks or months following discharge.
Our study shows that long-term outcomes of paediatric NP are good. Long-term impairment of pulmonary function is rare, even in children with severe complications (air leak or empyema). Only a few studies have assessed pulmonary function in children with complicated pneumonia, and even fewer in children with NP. The findings of these studies have been inconsistent and, based on our literature review, none have analysed long-term clinical outcomes and impact on lung function.
In relation to pneumonia with pleural effusion, Kohn et al. found abnormalities in lung function in 35% of patients a year after the episode of empyema (restrictive pattern in 19%, obstructive in 16%, normal in 65%).31 It is worth noting that surgery was performed in 58% of these patients, whereas in our sample none of the patients underwent a thoracotomy. On the other hand, the study by Cohen et al. described excellent outcomes of childhood empyema at the 1-year follow-up. In their study, only 2 of 34 patients had abnormal lung function results (obstructive pattern) 12 months after empyema.32 Honkinen et al. found that the long-term outcomes of children with parapneumonic empyema were also good. The spirometry was normal in 20 of the 25 patients, 2 patients had pre-existing asthma, 2 more patients had lung function values consistent with asthma, and 1 patient had a decreased lung volume resulting from the lung resection.33
No longitudinal studies have analysed pulmonary function in children with NP. To date, only 1 large case series has been published by Sawicki et al., but the follow-up was short-term. The authors identified a total of 80 patients with NP, of who 64 were seen at the hospital after discharge. The median duration of follow-up was 6 months, while in our study the median duration was 8.75 years. All patients in the series published by Sawicki had complete resolution of symptoms. Only 12 patients underwent pulmonary function testing: 8 had normal lung function, 3 had a mild obstructive pattern and 1 had a mild restrictive pattern.22 The authors concluded that more studies were required to have a better understanding on the functional outcomes of affected children.
Our study analysed long-term pulmonary function outcomes in 24 patients with a history of NP. The spirometry was normal, without an obstructive pattern, and there was only 1 patient with a FVC in the lower limit of normal.
Although the FEV1 and FVC were greater than the LLN, in most patients their values were in the z-score range of 0 to –1.64 (between the 5th and 50th percentiles). A total of 19 patients had FEV1 z-scores between –1.64 and 0, and 19 patients had FVC z-scores of less than zero. These patients had pulmonary function parameters in the normal range, but with negative z-score values.
A pulmonary function in the low range of normality may be associated with a higher risk of impaired pulmonary function in adulthood.8–10 Therefore, NP could be a risk factor for lung function decline in the long term, although studies with larger sample sizes are needed to verify this hypothesis. Adequate follow-up of these patients in adulthood is advisable.
Our study is limited by the small sample size and the lack of data on lung function before the episode of NP. We were unable to determine whether lung function in these patients was abnormal prior to the episode under study. We found only 1 article in the literature that assessed lung function in children following an episode of NP. This series was published by Sawicki et al. and included only 12 patients (fewer than our series).22
Another limitation of our study was the lack of a control group, although we compared the patients in our study with reference values for age, sex, height and ethnicity derived in a similar population based on data obtained from the GLI study. Therefore, there was a group without disease or a previous history of NP that had similar characteristics.14–17
In conclusion, despite the severity of the short-term morbidity of necrotizing pneumonia in children, the long-term clinical outcomes are good. However, a history of NP in childhood seems to be associated with minor changes in lung function several years after the episode. Follow-up of these patients is advisable on account of the potential decline in lung function in adulthood.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Bover-Bauza C, Osona B, Gil JA, Peña JA, Figuerola J. Resultados a largo plazo de la neumonía necrotizante. An Pediatr (Barc). 2021;95:298–306.