Ototoxicity occurs in different percentages in patients after treatment with platinum-based chemotherapy or cranial radiation therapy. The aim of this study was to present our experience in ototoxicity monitoring.
Material and methodsA review was made of the registry of paediatric cancer patients referred to the Children’s Hearing Loss Unit from 1999 to 2019.
ResultsOf the 46 patients referred to this unit, 41 had received platinum as part of their treatment, 17 patients underwent neurosurgery, and 18 patients received cranial radiation therapy. An anamnesis and otoscopy were performed on all of them, and the monitoring was carried out with tone-verbal audiometry and/or distortion products. Hearing loss was observed in eight patients (21.05% of patients referred for audiological follow-up) as a consequence of the treatment. It was impossible to determine the audiological situation in eight patients at the end of treatment. Hearing aid adaption was necessary in two patients. In coordination with Paediatric Oncology, a change from cisplatin to carboplatin due to bilateral grade two ototoxicity was considered appropriate during treatment in one patient.
ConclusionAdequate coordination with Paediatric Oncology is essential to carry out active surveillance for ototoxicity and to modify, if possible, the dosage or type of chemotherapy in case hearing is affected. In our experience, and following current recommendations, a pre-treatment assessment is usually performed, as well as monitoring during treatment, at the end of treatment, and annually thereafter due to the risk of a later development of hearing loss.
La ototoxicidad se presenta en diversos porcentajes según estudios tras el tratamiento con quimioterapia basada en platino y/o radioterapia craneal. El objetivo es mostrar nuestra experiencia en la monitorización de la ototoxicidad.
Material y métodosSe realizó una revisión del 1999 al 2019 en el registro de pacientes oncológicos pediátricos de nuestro hospital y remitidos a la Unidad de Hipoacusia Infantil.
Resultados46 pacientes fueron remitidos a nuestra unidad. 41 pacientes recibieron platinos como parte de su tratamiento, 17 pacientes fueron sometidos a una intervención neuroquirúrgica y 18 pacientes recibieron radioterapia craneal. A todos se les realizó una anamnesis y otoscopia, y la monitorización se llevó a cabo con una audiometría tono-verbal y/o productos de distorsión. Se objetivó una hipoacusia como secuela del tratamiento en ocho pacientes (21,05% de los pacientes remitidos para seguimiento audiológico). Fue imposible determinar la situación audiológica al finalizar el tratamiento en ocho pacientes. La adaptación audioprotésica fue necesaria en dos pacientes. En la coordinación con Oncología Pediátrica, se consideró oportuno el cambio de cisplatino por carboplatino por ototoxicidad importante durante el tratamiento en un único paciente.
ConclusiónEs imprescindible una adecuada coordinación con Oncología Pediátrica para realizar una vigilancia activa de la ototoxicidad y modificar, si es posible, la dosificación o el tipo de quimioterápico en caso de verse afectada la audición. En nuestra experiencia, y siguiendo las recomendaciones actuales, realizamos una valoración pretratamiento, una monitorización durante el tratamiento, al finalizarlo y después de forma anual por el riesgo de desarrollo diferido de una hipoacusia
Ototoxic drugs cause dysfunction and/or cellular degeneration of the tissues of the inner ear. Cochleotoxicity is defined as damage involving the auditory system resulting in neurosensory hearing loss and/or tinnitus.1 At present, more than 150 drugs are considered ototoxic. They include aminoglycosides, glycopeptides and macrolides, platinum-based antineoplastic drugs, loop diuretics, quinine and salicylates, among others.2 Ototoxicity is, as a rule, permanent. However, a mechanism for reversing hearing loss has been observed in some animals and there have been some cases in humans illustrating that early damage to the the marginal cells of the stria vascularis (for instance, with the use of cisplatin) can be reversed if healing processes are allowed to happen. If this is not the case, the increasing accumulation of the ototoxic agent precludes the possibility of recovery, resulting in outer hair cell death and permanent hearing loss.3
Advances in the treatment of paediatric cancer have improved the prognosis of affected patients, with a mean 5-year survival greater than 80% in developed countries. However, these improvements are tainted by the presence of long-term adverse effects of treatment.4–6 One of them is ototoxicity, which occurs in 50% of patients treated with platinum-based chemotherapy, cranial irradiation or both.4,7 It manifests as high-frequency hearing loss, usually accompanied by tinnitus.4,8 Ototoxicity may affect both children and adults, but children, especially the youngest, are more vulnerable to its effects, as hearing and language skills are still developing in these patients.9
Despite current recommendations, monitoring of ototoxicity in paediatric patients is still inadequate. A study found that only 72% of patients considered to be at risk underwent hearing tests after treatment and only 43% had full audiologic monitoring before, during, and after treatment.10 The fact that children with moderate to severe neurosensory hearing loss due to ototoxicity exhibit significant impairment in overall cognitive functioning, reading and quantitative reasoning at 5 years11 and that these sequelae could be prevented with adequate hearing loss treatment evinces the importance of audiologic care from initiation of cancer treatment.4
The aim of our study was to summarise our experience in the surveillance of ototoxicity in paediatric oncology patients in a tertiary care hospital over 20 years and explain the auditory function monitoring protocol and the long-term follow-up of hearing that derives from it, which have incorporated the technological and organizational advances of the past decade.
Material and methodsWe reviewed the register of oncology patients with solid tumours managed in the department of paediatrics of our hospital from 1999 to 2019 that were referred to the paediatric hearing loss unit for monitoring of ototoxicity. The study was approved by the Research Ethics Committee of the Principality of Asturias (Project No. 2020.287; June 8, 2020).
We conducted a retrospective and descriptive analysis of the clinical characteristics of patients and analysed their association with the development of ototoxicity during treatment or follow-up. We summarised the clinical and demographic characteristics of the patients using standard statistics. We analysed the correlation between ototoxicity and clinical variables by means of the chi square test with the software IBM SPSS Statistics, version 21 for Mac. Statistical significance was defined as a p-value of less than 0.05.
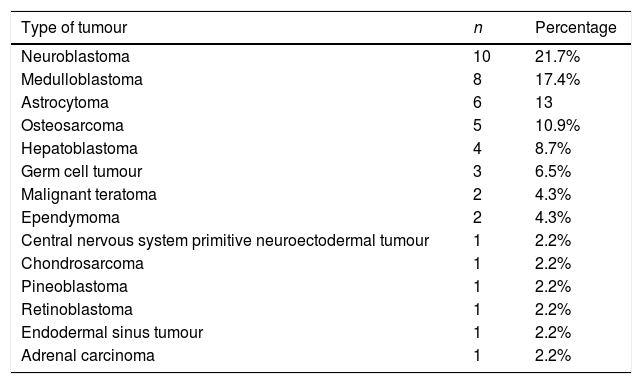
ResultsA total of 46 patients were referred to the paediatric hearing loss unit. The mean age at diagnosis was 6 years, and 24 patients were male (52%) and 22 female (48%). Of all patients, 41 did not have a history of interest, 1 had congenital hearing loss, 3 had congenital syndromes (Li-Fraumeni syndrome, Poland syndrome and Beckwith-Wiedemann syndrome) and 1 had coarctation of the aorta. Table 1 present the types of tumours present at diagnosis.
Types of tumours found at diagnosis.
| Type of tumour | n | Percentage |
|---|---|---|
| Neuroblastoma | 10 | 21.7% |
| Medulloblastoma | 8 | 17.4% |
| Astrocytoma | 6 | 13 |
| Osteosarcoma | 5 | 10.9% |
| Hepatoblastoma | 4 | 8.7% |
| Germ cell tumour | 3 | 6.5% |
| Malignant teratoma | 2 | 4.3% |
| Ependymoma | 2 | 4.3% |
| Central nervous system primitive neuroectodermal tumour | 1 | 2.2% |
| Chondrosarcoma | 1 | 2.2% |
| Pineoblastoma | 1 | 2.2% |
| Retinoblastoma | 1 | 2.2% |
| Endodermal sinus tumour | 1 | 2.2% |
| Adrenal carcinoma | 1 | 2.2% |
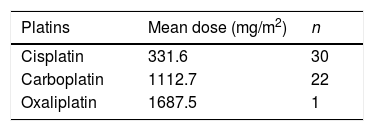
Treatment included platins in a total of 41 patients, 17 patients underwent brain surgery and 18 patients cranial irradiation. Table 2 presents the combination therapies administered to the patients. Table 3 presents the mean doses of platins administered to the patients. There were 36 patients who received cisplatin and/or doses of carboplatin greater than 1500 mg/m2 (cisplatin, 17 patients; carboplatin at doses >1500 mg/m2, 4 patients; cisplatin and carboplatin, 15 patients). When it came to radiation therapy, the mean dose was 57.1 Gy, and all patients received cranial irradiation at doses greater than 30 Gy.
Mean doses of platin administered to patients.
| Platins | Mean dose (mg/m2) | n |
|---|---|---|
| Cisplatin | 331.6 | 30 |
| Carboplatin | 1112.7 | 22 |
| Oxaliplatin | 1687.5 | 1 |
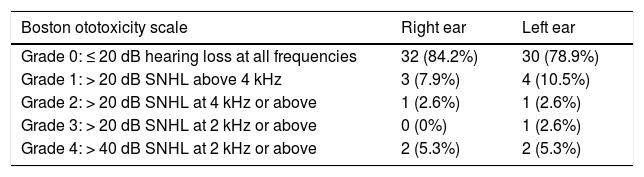
An anamnesis and otoscopy examination were performed in all patients referred to the paediatric hearing loss unit. Monitoring involved the use of pure tone-speech audiometry, distortion product otoacoustic emissions or both. Patients under 2 years were evaluated with behavioural observation audiometry adapted for their age. Twenty-eight patients were referred for audiologic evaluation before initiation of ototoxic treatments, of who only 1 had pre-existing hearing loss. However, hearing loss was not evaluated at baseline in the remaining 18 patients. During treatment and follow-up, there was evidence of hearing loss attributable to treatment-related ototoxicity in 8 patients (2 aged less than 2 years, 3 aged 2–6 years and 3 aged more than 6 years). We were unable to determine the auditory status at the end of treatment in 8 patients (3 deceased and 5 that did not attend scheduled follow-up visits). Thus, 21.05% of patients with adequate audiologic follow-up had hearing loss. We quantified ototoxicity in each ear using the Society of Pediatric Oncology(SIOP)/Boston Ototoxicity grading scale (Table 4). Only 1 patient reported bilateral tinnitus as a sequela of treatment. Although hearing loss was not detected in audiometric tests, the patient did report bilateral tinnitus after completing treatment. Tinnitus sound therapy was recommended to the patient, who underwent evaluations at regular intervals and reported good tolerance to the sounds. One female patient presented pseudohypoacusis with abnormal audiometric thresholds that did not match observations in conversation or the normal results of objective evaluations. Hearing aids were recommended to 2 patients due to ototoxicity classified as grade IV in the Boston scale. None of the patients required rehabilitation with speech therapy or underwent cochlear implantation. In 1 patient, the decision was made in agreement with the paediatric oncology team to switch from cisplatin to carboplatin due to development of bilateral grade 2 ototoxicity during treatment.
Boston SIOP ototoxicity scale.
| Boston ototoxicity scale | Right ear | Left ear |
|---|---|---|
| Grade 0: ≤ 20 dB hearing loss at all frequencies | 32 (84.2%) | 30 (78.9%) |
| Grade 1: > 20 dB SNHL above 4 kHz | 3 (7.9%) | 4 (10.5%) |
| Grade 2: > 20 dB SNHL at 4 kHz or above | 1 (2.6%) | 1 (2.6%) |
| Grade 3: > 20 dB SNHL at 2 kHz or above | 0 (0%) | 1 (2.6%) |
| Grade 4: > 40 dB SNHL at 2 kHz or above | 2 (5.3%) | 2 (5.3%) |
SNHL, sensorineural hearing loss.
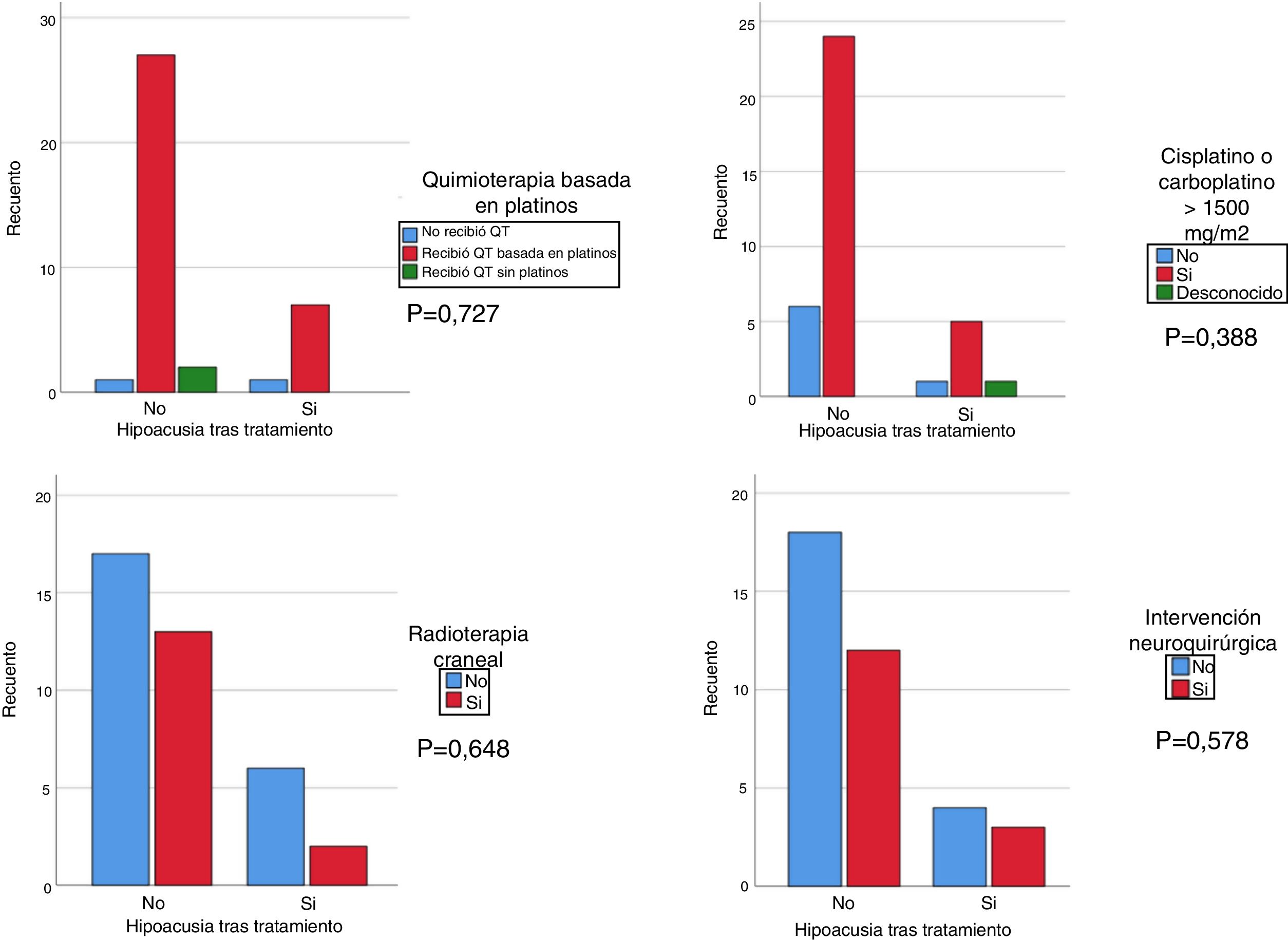
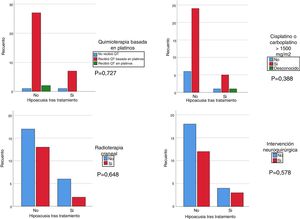
We used the chi square test to assess the correlation of hearing loss after treatment with treatment with cisplatin at any dose and/or carboplatin at a dose greater than 1500 mg/m2, cranial irradiation at a dose greater than 30 Gy and/or brain surgery. Although the results were not statistically significant, we found that patients treated with platins, cranial irradiation and cisplatin and/or carboplatin at doses greater than 1500 mg/m2 had more severe hearing loss compared to patients not treated with cisplatin or treated with doses of less than 1500 mg/m2 and/or not treated with cranial irradiation (Fig. 1). We analysed the association of hearing loss with age comparing age groups (<2 years, 2–6 years, >6 years) and did not find statistically significant differences.
DiscussionFrom the time, screening programmes for early detection of hearing loss in paediatric patients were widely implemented, the management of hearing loss in this population has improved thanks to important advances in technology (diagnostic and therapeutic) and the creation of paediatric audiology units, responsible for confirming hearing loss detected in screening, investigating its aetiology and providing early intervention. At the same time, patients with late-onset hearing loss have also benefitted from this progress, as paediatricians and otorhinolaryngologists have become aware of the importance of early detection and treatment of this health problem in general and of ototoxicity in particular, especially in oncology patients.
To provide adequate audiologic care and minimise the complications of hearing loss, including impairments in speech, social skills, affect and learning, audiologic monitoring of patients that are going to receive ototoxic treatment is of the essence.
Cisplatin-related ototoxicity results from damage to the organ of Corti or, more specifically, the degeneration of stereocilia in outer hair cells, the stria vascularis and the spiral ganglion.12 Damage to outer hair cells precedes damage to inner hair cells and is more pronounced in the scala media of the cochlea, which explains the bilateral audiometric presentation with greater hearing loss in high frequencies that gradually extends to lower frequencies with prolonged exposure.13 Children under 5 years (probably due to cochlear and vascular immaturity) and children treated with cumulative doses of cisplatin greater than 400 mg/m2 are at higher risk. Hearing loss is usually permanent, bilateral and symmetrical (although cases of asymmetrical hearing loss have also been reported), and usually develops during treatment or shortly after its completion, although it may also progress slowly over time, even 20 years after completing treatment.14 Carboplatin is a second-generation platinum derivative, and while it is considered a less-toxic analogue of cisplatin, it can have an ototoxic effect on the cochlea, especially when administered at high (myeloablative) doses.15 The incidence of ototoxicity ranges from 0% to 16.7%, and we ought to highlight that the onset of hearing loss is often delayed (developing a mean of 14 months after the last dose).16 Cranial irradiation is frequently combined with cisplatin for treatment of some forms of cancer, and this combination carries a higher risk of hearing loss. The diagnosis of irradiation-related hearing loss is more challenging, as it may manifest as conductive hearing loss (due to damage to the outer and/or middle ear), mixed hearing loss, neurosensory hearing loss or retrocochlear hearing loss. In the two latter forms, the impairment results from the degeneration of hair cells, supporting cells in the organ of Corti and/or vestibulocochlear nerve fibres.17
The audiogram typically detects greater impairment in the perception of high frequencies, and hearing loss may develop gradually, even years after exposure. Greater severity is associated with younger age, combined therapy with cisplatin and the irradiation dose, with a maximum dose of 35 Gy recommended for children to minimise ototoxicity.18,19
In the sample under study, we found that 21.05% of the children developed hearing loss secondary to treatment with platins, cranial irradiation and/or brain surgery. Previous studies have reported higher proportions of around 50%,7,9,20,21 but there is considerable variation (ranging from 1.7% to 90.1%). This heterogeneity reflects differences between studies in the definition of hearing loss, the tests used for diagnosis, patient characteristics, the cancer treatments used, the administration of other ototoxic drugs and the duration of follow-up.22
It is essential that these patients be referred for audiologic evaluation, given the importance of early detection and treatment of hearing loss in childhood, especially in circumstances in which the likelihood of hearing loss is high. Close monitoring for detection of hearing loss is especially recommended in children treated with cisplatin, alone or combined with carboplatin at high doses (>1500 mg/m2), or with cranial irradiation at a dose of 30 Gy or greater.
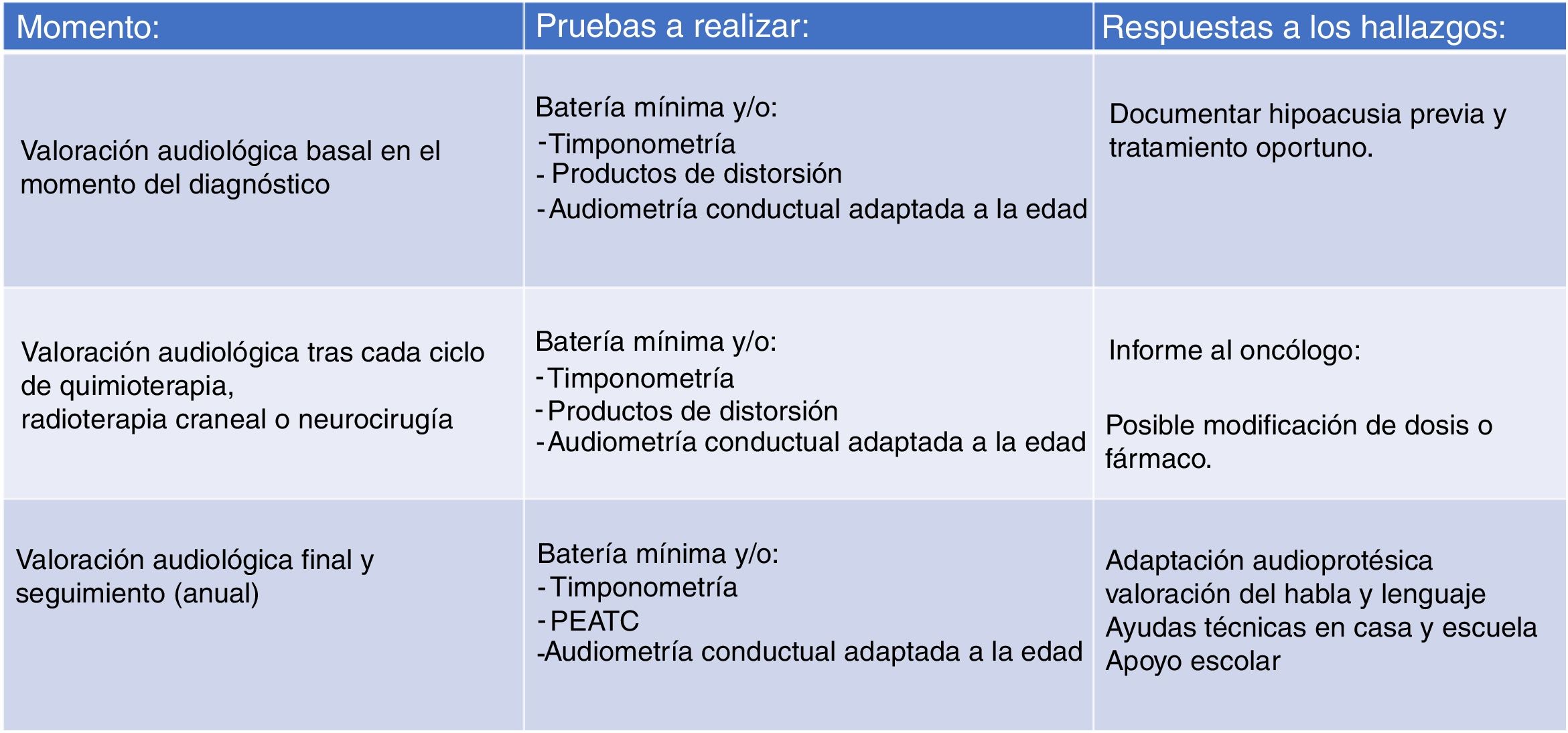
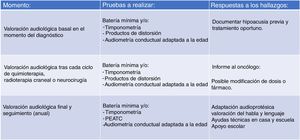
Due to the large number of patients in our case series that did not have a baseline audiologic evaluation (before initiating treatment) or were lost to follow-up, we believed that the monitoring protocol had to be modified, and we proposed the protocol that is currently in use (Fig. 2). We aim to facilitate access to audiologic evaluations by making scheduling in the paediatric hearing loss unit as flexible as possible (on-demand scheduling of evaluations if the child is visiting the hospital for other tests or specialists) so that every patient set to receive potentially ototoxic treatment undergoes an audiologic evaluation before treatment starts. It is also important to take into account that the condition of some children may preclude them from participating in behavioural audiologic evaluations, so the system used to grade hearing loss has to be flexible enough to accommodate this eventuality.
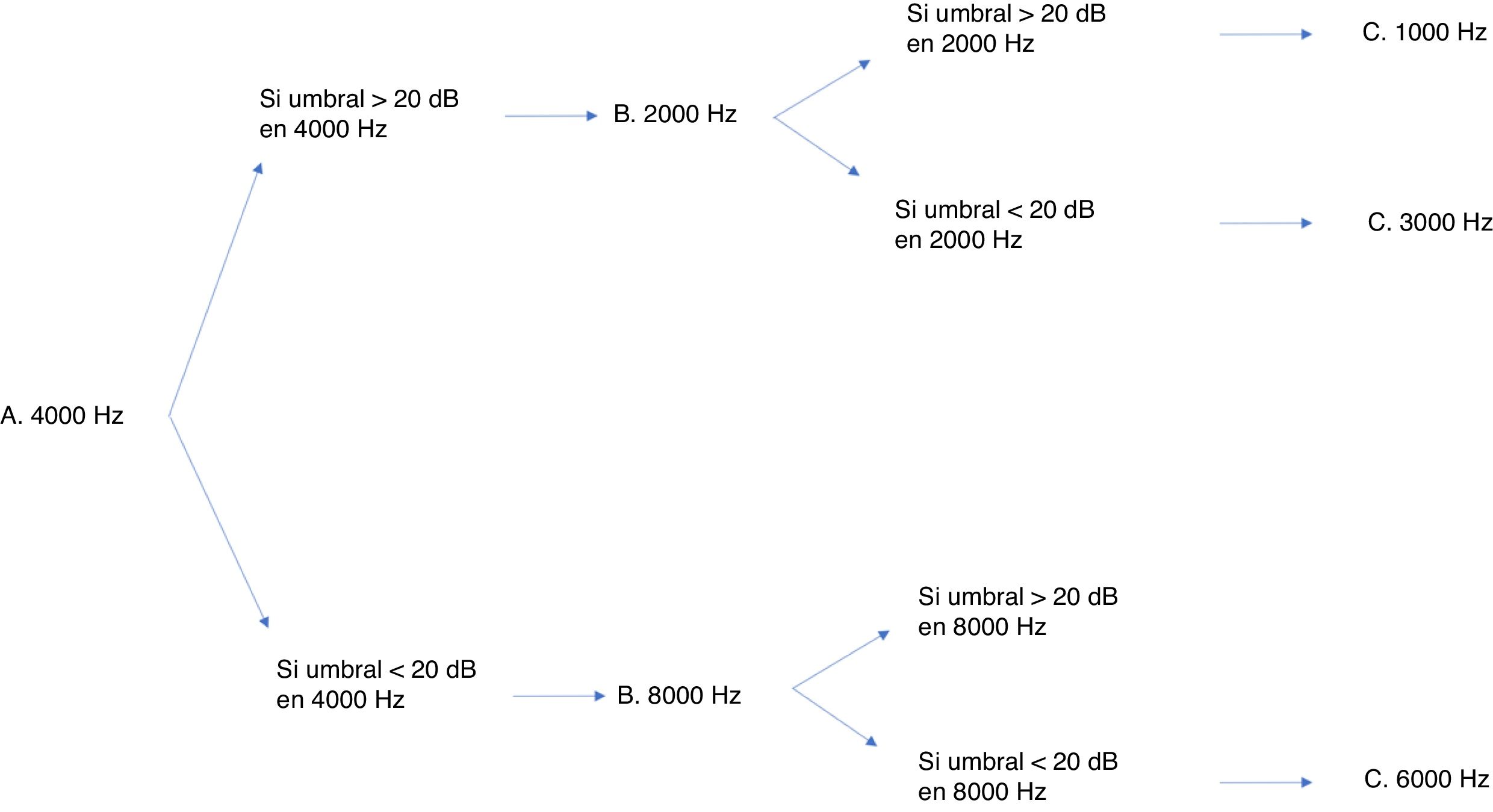
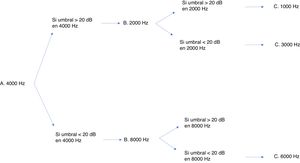
Many protocols have been proposed for monitoring ototoxicity, but since uniformity is important, we chose the international consensus-based Boston SIOP grading scale.23 Although having baseline test results is ideal, this protocol does not require it, so it leaves some leeway for cases in which the condition of the patient does not allow a baseline evaluation. In addition, this scale is sensitive to small changes in hearing in the high-frequency range, and requires measurement of thresholds for very few frequencies to be able to grade hearing loss.24 While this grading scale only requires assessment with a few frequencies, a full audiologic evaluation is still recommended if the condition of the patient allows it. Fig. 3 presents the minimum test battery required for the Boston SIOP scale. Other audiologic tests, such as evoked otoacoustic emissions or evoked auditory brainstem potentials, cannot replace behavioural audiometric testing.25 The evoked auditory brainstem potentials are a possible exception, although their performance in the context of chemotherapy protocols has yet to be validated. Distortion product evoked otoacoustic emissions can detect changes in cochlear function before a decline is detectable in the audiogram,26 so they have proven effective in detecting cochlear damage. However, as is the case with evoked potentials, this approach has yet to be validated. In our unit, distortion product otoacoustic emissions have been used effectively to document hearing loss. In the case of an older girl that exhibited significant hearing loss, they proved that the patient had pseudohypoacusis, as the distortion product values were normal, thereby preventing unnecessary prescription of hearing aids.
The protocol currently applied in our unit, based on the Boston SIOP grading system, is structured in 3 levels (Fig. 2). The first involves the baseline audiological evaluation, which should be performed, if possible, before initiation of cancer treatment. The second level involves monitoring with audiometric testing after each cycle of chemotherapy or any other potentially ototoxic treatment (irradiation or brain surgery) in order to document treatment-related hearing loss and inform the oncologist. Lastly, the third level involves planning the audiologic follow-up after completion of cancer treatment, including speech and language evaluations. Follow-up evaluations will be conducted on completing treatment and annually thereafter in children aged less than 6 years and between 6 and 12 years, and every 5 years in adolescents aged more than 12 years, or whenever there is a change in the clinical presentation.
For the benefit of patients in whom hearing impairment is documented, an agile care pathway should be established to provide early audioprosthetic evaluation and effective fitting of hearing aids, speech rehabilitation and support in the school setting.
Grading systems that are easy to apply are important in clinical practice, but also in research, for instance, in the search of otoprotective agents. Systems that are both sensitive and can be applied uniformly are required to document outcomes and to allow comparison of the multiple studies on the subject published in the literature. There are also studies that seek to identify individual risk factors for hearing loss secondary to ototoxicity. Fifty percent of children treated with platins eventually develop hearing loss, but it would be very difficult to identify the factors that protect the remaining patients from its development without performing multicentre studies with large enough samples and establishing a standardised system to register outcomes.24 Due to the heterogeneity of results from genetic association studies performed so far, the evidence seems insufficient to recommend screening for specific markers.27
The development of tinnitus during or after treatment is a largely neglected issue, despite an estimated incidence of up to 40% in these patients. This symptom, which can have a significant impact on quality of life, is difficult to assess and detect. Version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute includes tinnitus as one of the ear and labyrinth disorders associated with chemotherapy.28 The most recent and widely used instrument for assessment of tinnitus is the tinnitus functional index (TFI) is designed to grade the severity of tinnitus and assess the response to treatment, although this questionnaire can only be used in older children and adolescents.29 The management in paediatric patients of hearing impairments associated with the serious condition involved in an oncological illness and its treatment highlights that there are areas in which the collaboration of different specialists can help improve outcomes and alleviate the severe sequelae that hearing loss can cause without adequate treatment. In addition to ensuring normal development in the future, it can significantly improve the potential fragility of families by managing the problem in the solid care circuit promoted by the widespread implementation of newborn hearing screening and the creation of paediatric hearing loss units and early intervention and school integration programmes.
In conclusion, we consider agile care coordination between departments essential (paediatrics, radiation therapy, pharmacy, neurosurgery) to actively monitor ototoxicity in this patient population and, when possible, adjust the dose of the chemotherapy agent in case hearing impairment is detected. In our unit, and in adherence with the most recent recommendations, we evaluated patients before initiating ototoxic treatment, followed by audiologic monitoring during and after treatment and annual evaluations thereafter due to the risk of late-onset hearing loss.
FundingThe authors did not receive any funding to produce this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sánchez-Canteli M, Núñez-Batalla F, Martínez-González P, de Lucio-Delgado A, Antonio Villegas-Rubio J, Gómez-Martínez JR, et al. Ototoxicidad en pacientes oncológicos: experiencia y propuesta de un protocolo de vigilancia. An Pediatr (Barc). 2021;95:290–297.