The minimally invasive technique known as MIST (Minimally Invasive Surfactant Therapy), is a method which allows surfactant to be administered to a patient connected to non-invasive respiratory support. Use of this therapy is growing in Neonatal Units, as it reduces the intubation rate and the pathology associated with intubation and allows surfactant to be administered to patients in need.
Patients and methodsIn 2013 and 2014 in the Hospital General Universitario de Elche surfactant was delivered using this method to 19 patients, 5 of whom had a gestational age of 28 or less weeks. Data were compared with a historical cohort consisting of 28 patients with Respiratory Distress Syndrome treated initially with non-invasive respiratory support.
ResultsNo incidents were recorded that caused interruption of administration. A reduction in the fraction of inspired oxygen was observed in all cases after surfactant administration. Fewer intubations in the first 72h of life were found in the treatment group compared to the control group (42% vs. 54%).
DiscussionThe experience in the Hospital General Universitario de Elche shows that administration of surfactant using the MIST technique is a reproducible method of treatment, which allows surfactant distribution during spontaneous breathing with non-invasive respiratory support.
La administración de surfactante mediante técnica mínimamente invasiva, conocida como MIST por sus siglas en inglés (Minimal Invasive Surfactant Therapy), es un procedimiento que permite administrar el surfactante estando el paciente conectado a ventilación no invasiva. Cada vez más utilizada en las unidades neonatales, permite reducir el número de intubaciones y la patología asociada a la misma, a la vez que no se priva de la administración de surfactante a los pacientes que lo necesitan.
Pacientes y métodosEn los años 2013 y 2014 en el Hospital General Universitario de Elche se administró surfactante mediante técnica mínimamente invasiva en 19 pacientes, 5 de ellos con una edad gestacional igual o menor de 28 semanas al nacimiento. Se realiza comparación con una cohorte histórica de 28 pacientes con distrés respiratorio neonatal que fueron inicialmente tratados con soporte respiratorio no invasivo.
ResultadosNo se registraron complicaciones que obligaran a detener la técnica. Se observó en todos los casos una disminución de las necesidades de la fracción inspirada de oxígeno. El número de intubaciones fue menor en el grupo MIST respecto al grupo control (42% vs. 54%).
DiscusiónLa experiencia recopilada en el presente estudio muestra que la administración de surfactante con técnica MIST es un procedimiento reproducible que permite una buena distribución del surfactante en ventilación no invasiva.
The addition of exogenous surfactant to treat hyaline membrane disease (HMD) has unquestionably been revolutionary. There are numerous studies showing how administration reduces mortality and morbidity among patients,1 while mechanical ventilation has been associated with an increase in morbidity.2
Studies in animal models show that distribution of exogenous surfactant and incorporation into the endogenous metabolism is improved when surfactant is administered during spontaneous breathing with nasal continuous positive airway pressure instead of during conventional mechanical ventilation (MV).3
One alternative to administering surfactant and avoiding MV is the intubation–surfactant–extubation (INSURE) method. With this method, surfactant is administered after intubation immediately followed by extubation and non-invasive ventilation (NIV). Some studies have shown that this method reduces incidence of morbidity, air-leak syndrome, and the need for MV.4,5 Despite these advantages, it should not be forgotten that the INSURE method requires the patient to be intubated, with the risks inherent to this procedure, and distribution of surfactant under intermittent positive pressure delivery is less effective than under spontaneous breathing. Added to this are the difficulties involved in extubation due to the need to sedate the infant once surfactant has been administered.6
MIST was developed to facilitate administration of exogenous surfactant without the need for MV. MIST techniques include nasopharyngeal instillation,7 administration through a laryngeal mask,8 aereosolisation9 and techniques requiring tracheal catheterisation. Of these, the most widely used today is tracheal catheterisation, due to its ease of use, the possibility of administering surfactant quickly, and its effective distribution. Various methods have been developed using the tracheal catheterisation procedure.
Kribs et al. suggested the administration of surfactant using a technique known as the Cologne method, which consists of introducing a 4–5 FG nasogastric tube (NGT) in the trachea with the aid of Magill forceps. After cannulating the trachea, the laryngoscope is removed, surfactant is administered, and then the NGT is removed, maintaining the patient on NIV at all times. This technique can be challenging for professionals unaccusomed to using forceps.10–12 A variant of the Cologne method is known as Take Care, which also uses an FG NGT but does not require the use of Magill forceps.
The Hobart method was devised with the aim simplifying the technique; it was developed by Dargaville and does not use Magill forceps. This is achieved by using a 16G angiocatheter, more rigid than an NGT, which allows greater control of the direction of the catheter, introducing it directly through the vocal cords, and administering the surfactant while maintaining NIV.13
Studies comparing surfactant administration by means of the INSURE method with MIST techniques have shown that MIST reduces cases of bronchiopulmonary dysplasia, measured as an NNT of 10 in the clinical trial published by Kanmaz et al.6
Patients and methodsThis is a retrospective observational study analysing data collected from patients who have received surfactant by means of the MIST technique using an angiocatheter (Hobart method).
A total of 19 patients receiving surfactant using the MIST technique in 2013 and 2014 in the Neonatology Unit of a tertiary hospital (MIST group) were included. Patients are classified into 2 groups based on gestational age (GA) at birth: 5 patients with a GA at birth between 25 and 28 weeks, and a second group with 14 patients with a GA at birth between 29 and 34 weeks (Table 1).
Characteristics of patients.
| MIST | Controls | |||||
|---|---|---|---|---|---|---|
| TotalN=19 | GA: 25–28N=5 | GA: 29–34N=14 | TotalN=28 | GA: 25–28N=5 | GA: 29–34N=23 | |
| GA | 30 (5) | 26 (1) | 31 (1) | 32 (4) | 28 (0) | 32 (1) |
| Weight (g)a | 1484±443 | 928±135.72 | 1682.86±324.75 | 1690±549.04 | 1053±101.16 | 1882±472.84 |
| Lung maturation | 100% | 100% | 100% | 75% | 100% | 69.5% |
| Apgar 1min | 7 (3) | 7 (1) | 8 (4) | 8 (2) | 6 (0) | 8 (0) |
| Apgar 5min | 8 (2) | 8 (1) | 8 (3) | 9 (2) | 8 (0) | 8 (0) |
| Hours of life surfactant | 0.75 (7.6) | 0.5 (0.3) | 1 (15.5) | 2.5 (5.5) | 2 (0) | 3 (0) |
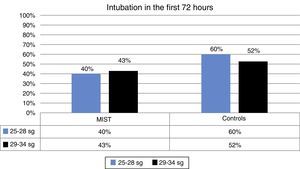
| Intubation <72h | 42% | 40% | 43% | 54% | 60% | 52% |
| FiO2 before | 0.3 (0.13) | 0.3 (0.21) | 0.3 (0.13) | 0.35 (0.1) | 0.3 (0) | 0.4 (0.1) |
| FiO2 after | 0.21 (0.02) | 0.21 (0.18) | 0.21 (0) | 0.23 (0.04) | 0.21 (0) | 0.25 (0) |
Medians are shown with the interquartile range in brackets.
Differences between groups did not show statistical significance following their analysis (p>0.05).
This technique is used in premature infants with respiratory distress who have suspected surfactant deficiency and who need a fraction of inspired oxygen (FiO2) equal to or greater than 0.3, in spite of non-invasive respiratory support. NIV is supplied with the Infant Flow Sipap® respirator, with binasal interface, in CPAP mode or in continuous pressure with cycled ventilation mode (BiPhasic®). NIV is considered optimised in the CPAP mode when pressures of 5–6cmH2O are reached, and in the BiPhasic® mode with inspiratory pressures of 8–10cmH2O, setting an inspiratory time of 0.5s. The management of these patients is based on the recommendations included in the departmental clinical guidelines.
During the technique, the patient is on NIV at all times. Placement of a continuous suction NGT to check for surfactant reflux and passage to the digestive system and sedation prior to the procedure is optional. No pre-medication was used for any patients in our series.
Surfactant is administered via direct laryngoscopy through an Angiocath™ 16 GA catheter, after removing the guidewire. The catheter is introduced to a depth of 1–1.5cm into the trachea, and then the laryngoscope is removed, holding the catheter to immobilise it. Surfactant of porcine origin (Curosurf®) is administered at this point, at a dose of 200mg/kg in 2–4 boluses of 15–30s each. The catheter is removed after this, and NIV continues.
Different variables were collected from the patients who received surfactant following the procedure described above. These included family situation, GA, weight, hours of life at the time of administration of the first surfactant dose, number of attempts, number of technique repetitions, number of incidents and whether these involved an interruption of the technique (apnoea, oxygen desaturation, surfactant reflux), whether intubation was required in the first 72h after surfactant administration and the reason for intubation. The FiO2 prior to the administration of surfactant and the ratio required by the patient 1 hour after the procedure was noted.
The technique was interrupted in cases of bradycardia, desaturation or surfactant reflux. Each trachea cannulation attempt was considered to have a maximum duration of 30s and the maximum number of attempts was defined as 3.
A cohort of patients diagnosed with HMD in 2011 and 2012 was chosen as the control group. They had initially been managed with non-invasive respiratory support (nasal CPAP or nasal bilevel positive pressure mode) (control group). During this period in our Unit surfactant was not administered using minimally invasive techniques. This group included a total of 28 patients. Five patients had a GA at birth between 25 and 28 weeks and 23 patients had a GA at birth between 29 and 34 weeks. In these patients, respiratory distress was initially managed with NIV, resorting to MV and surfactant administration through an endotracheal tube in cases of failed NIV.
Optimised NIV is considered to have failed after administration of surfactant in the MIST group, or prior to administration of surfactant in control group patients, when the patient presented a need for FiO2≥0.3 and/or sustained hypercapnia (PaCO2>60mmHg) with acidosis (pH<7.25), as well as severe respiratory difficulty and/or frequent (>3 in 1h requiring vigorous stimulation) or serious (requiring ventilation with self-inflating bags) apnoea.
In our Unit, intubation is performed in the case of failure of NIV, contraindication for non-invasive mechanical ventilation, or compromised gas exchange due to absent stimulus or decreased muscle capability.
Data were analysed with SPSS v.22 software. The Chi-square test, the Mann–Whitney U, and the T-test were used for independent samples based on sample distribution.
ResultsA total of 19 patients received surfactant using the MIST technique with angiocatheter. All (100%) study patients received lung maturation therapy with betamethasone compared with 75% of patients in the control group.
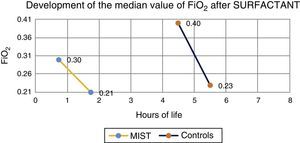
Median prior to administration of surfactant was 0.3 in MIST group patients and 0.4 in the control group. One hour after the procedure, median was 0.21 in those treated with the MIST technique, and 0.23 in the control group (Fig. 1).
The delay in administration of surfactant varied between patients in the MIST group and the control group. In the MIST group, surfactant was applied at a median of 45min, whereas in the control group it was applied at 4.5h. In the MIST group, 60% of neonates with GA equal to or lower than 28 weeks and 25% of those with greater GA received surfactant early, at 30min of life, with cannulation of a vascular access performed after surfactant administration. In the over-28 weeks group, early administration of surfactant was performed at a GA of 30 weeks.
Surfactant administration in the control group was performed at 1.86h on average in patients with a GA of 28 weeks or less, and at 10.9h on average in patients with a GA of over 28 weeks. The patient in this group who received surfactant at the earliest time did so at 40min of life.
As for incidents, 2 patients had surfactant reflux, which forced a second attempt to complete the dose in both cases. Another patient also required a second attempt because the technique had to be stopped due to oxygen desaturation, which required ventilation with intermittent positive pressure. The procedure was performed in all patients. No other incidences were recorded.
During the first 72h no intubation was required in 58% of the patients in the MIST group compared with 42% in the control group. This difference was not statistically significant (Fig. 2).
An analysis of the medians of other variables was conducted, such as length of stay (days) in the Neonatal Intensive Care Unit (14 days MIST group, 13 days control group), oxygen therapy days (2.5 days MIST group, 1.5 days control group), respiratory support days (6.5 days MIST group, 5.5 days control group), MV days (0 days MIST group, 1 day control group; p=0.228) and days on non-invasive respiratory support (5.5 days MIST group, 4 days control group; p=0.411), with no statistically significant differences.
There were no differences when analysing the incidence of mortality and of pathologies such as bronchiopulmonary dysplasia, patent ductus arteriosus, pneumothorax, necrotising enterocolitis, intraventricular haemorrhage, and premature retinopathy, possibly due to the reduced size of the sample.
DiscussionOur findings are consistent with those of other studies on MIST with angiocatheter (Hobart method),13,14 which is a safe and effective technique, reducing the need for FiO2. Because this is such a minimally invasive technique involving no intubation or venous access for drug delivery, administration of surfactant can start earlier than with other methods.
Spanish hospitals have considerable experience in the administration of surfactant with other minimally invasive techniques, recording good results. The study published by Aguar et al.14 describing the SONSURE method, in which surfactant was administered by means of an NGT with the aid of Magill forceps is a case in point.
In our series, in the MIST group, surfactant administration was started later in the over-28-week GA group than in the under-28-week GA group. This was because start of the procedure was delayed until the patient fulfilled the requirements for surfactant administration. Specifically, 3 of these patients did not meet these requirements until after 20h of life.
Compared to the control group, surfactant was administered earlier in the MIST group. The reason is that with this technique, it is possible to separate the requirements of surfactant administration from the requirements of invasive respiratory support.
Although MIST patients required less intubation, difference between the MIST and control groups in this respect were not significant, possibly due to the small sample size.
In the MIST group, 42% of patients required intubation, half of them due to increased oxygen needs. We believe, on the basis of prior studies justifying a repeat of surfactant dose,1 that a second administration of surfactant by means of the MIST technique might have avoided up to 4 intubations in the group of patients treated with the study technique, which would imply no intubation in 79% of the sample.
A total of 54% of patients were intubated in the control group. Of these, 53% were only intubated to administer surfactant due to increased need of FiO2; therefore, the possibility of administering surfactant without invasive respiratory support could have prevented intubation in 75% of the sample.
Our findings should be interpreted with caution, due to the low incidence of complications and reduced sample size. The retrospective design of the study could have led to the exclusion of some data. Nevertheless, no significant complications were recorded, and the procedure was completed in all cases.
Prospective comparative studies with a larger number of patients are needed to draw conclusions on the effectiveness of the MIST technique, and the potential benefits (less need for invasive mechanical ventilation and oxygen therapy, and lower risk of bronchiopulmonary dysplasia) reported in other studies.6
The MIST technique is a major step in the treatment of surfactant deficiency and in the comprehensive care of premature neonates. It is a minimally invasive technique that respects the integrity of the neonate's respiratory system, and which does not rely on MV, with all its inherent risk, for administration. This prevents undue delays in starting surfactant therapy. The experience developed in this centre has allowed us to protocolise the technique, defining prophylactic indications in premature infants with a GA equal to or lower than 28 weeks and therapeutic indications in neonates presenting respiratory distress and/or FiO2 requirements equal to or greater than 0.3 that do not improve with NIV in the context of suspected surfactant deficiency. We also suggest repeating MIST therapy on patients who continue to meet surfactant requirements after the first dose in order to avoid intubation.
Conflicts of interestThe authors state there is no conflict of interest.
Please cite this article as: Canals Candela FJ, Vizcaíno Díaz C, Ferrández Berenguer MJ, Serrano Robles MI, Vázquez Gomis C, Quiles Durá JL. Terapia con surfactante con técnica mínimamente invasiva: experiencia en un hospital terciario. An Pediatr (Barc). 2016;84:79–84.