Congenital central hypoventilation syndrome (CCHS) is a very rare genetic disease. In 2012 the European Central Hypoventilation Syndrome (EuCHS) Consortium created an online patient registry in order to improve care.
AimTo determine the characteristics and outcomes of Spanish patients with CCHS, and detect clinical areas for improvement.
Materials and methodAn assessment was made on the data from Spanish patients in the European Registry, updated on December 2015.
ResultsThe Registry contained 38 patients, born between 1987 and 2013, in 18 hospitals. Thirteen (34.2%) were older than 18 years. Three patients had died. Genetic analysis identified PHOX2B mutations in 32 (86.5%) out of 37 patients assessed. The 20/25, 20/26 and 20/27 polyalanine repeat mutations (PARMs) represented 84.3% of all mutations. Longer PARMs had more, as well as more severe, autonomic dysfunctions. Eye diseases were present in 47%, with 16% having Hirschsprung disease, 13% with hypoglycaemia, and 5% with tumours. Thirty patients (79%) required ventilation from the neonatal period onwards, and 8 (21%) later on in life (late onset/presentation). Eight children (21%) were using mask ventilation at the first home discharge. Five of them were infants with neonatal onset, two of them, both having a severe mutation, were switched to tracheostomy after cardiorespiratory arrest at home. Approximately one-third (34.3%) of patients were de-cannulated and switched to mask ventilation at a mean age of 13.7 years. Educational reinforcement was required in 29.4% of children attending school.
ConclusionThe implementation of the EuCHS Registry in Spain has identified some relevant issues for optimising healthcare, such as the importance of genetic study for diagnosis and assessment of severity, the high frequency of eye disease and educational reinforcement, as well as some limitations in ventilatory techniques.
El síndrome de hipoventilación central congénita (SHCC) es una enfermedad genética muy rara causada por mutaciones en PHOX2B; en 2010 se creó el Consorcio Europeo del Síndrome de Hipoventilación Central, que en 2012 implantó un Registro online de pacientes para optimizar su cuidado.
ObjetivoConocer las características y la evolución de los pacientes españoles con SHCC y detectar áreas de mejora.
Materiales y métodoSe analizaron los datos actualizados en diciembre del 2015 de los pacientes españoles del Registro europeo.
ResultadosSe registró a 38 pacientes, nacidos entre 1987 y 2013, procedentes de 18 hospitales. El 34,2% eran mayores de 18 años. Han fallecido 3 pacientes. Aportaban estudio del gen PHOX2B 37 (97,3%), 32 (86,5%) con mutación. Los genotipos 20/25, 20/26 y 20/27 representaron el 84,3% de las mutaciones. Las disautonomías fueron más frecuentes y graves en portadores de genotipos con mayores expansiones de polialaninas. El 47% de pacientes asociaba alteraciones oculares, el 16% Hirschsprung, el 13% hipoglucemias y el 5% tumores. Treinta pacientes (79%) debutaron en el periodo neonatal y 8 (21%) posteriormente (inicio/diagnóstico tardío). Ocho niños (21%) recibieron inicialmente ventilación domiciliaria con mascarilla; 5 eran lactantes con comienzo neonatal, 2 de ellos precisaron cambio a traqueostomía tras presentar parada cardiorrespiratoria; ambos tenían mutaciones graves. Han sido decanulados y transferidos a mascarilla el 34,3% de los pacientes (edad media: 13,7 años). El 29,4% de los niños escolarizados precisaron refuerzo educativo.
ConclusiónLa implementación del Registro en España de pacientes con SHCC ha permitido identificar aspectos relevantes para optimizar sus cuidados, tales como la importancia del estudio genético para el diagnóstico y la estimación de gravedad, la frecuencia elevada de alteraciones oculares y de necesidad de refuerzo educativo, y algunas limitaciones de las técnicas ventilatorias.
Congenital central hypoventilation syndrome (CCHS), also known as “Ondine's curse”, is a very rare genetic disorder of the autonomic nervous system (ANS) characterised by the loss of autonomic control of respiration.1,2 It typically manifests during non-REM sleep or, in the most severe cases, throughout the sleep cycle and even during waking hours. It may be associated with other ANS dysregulation disorders (disturbances in the automatic control of heart rate,3 gastrointestinal motility and automatic eye movements,4,5 Hirschsprung disease6 or neural crest tumours7,8).
It is caused by heterozygous mutations in the paired-like homeobox 2B gene (PHOX2B), which plays a key role in the development of the ANS during embryogenesis.9,10 The most frequent mutations (90% of cases) correspond to what are known as polyalanine repeat expansion mutations (PARMs), which consist in expansions of the segment of 20 alanine residues located in exon 3 of PHOX2B; these expansions have variable length, and genotypes have been found with lengths ranging from 20/24 to 20/33. The remaining 10% of identified mutations are nonpolyalanine repeat expansion mutations (NPARMs), missense, nonsense or frameshift mutations11 or, more rarely (<1%), deletions or duplications of exon 3 or the entire gene.12 Most cases correspond to de novo mutations, although up to 25% of asymptomatic parents may have somatic mosaicism.13 Genetic testing confirms the diagnosis and provides a measure of severity, as mutations with shorter expansions (20/24 and 20/25) have variable penetrance and may even manifest with a normal phenotype, while PARMs involving larger expansions (20/27 and 20/33) are associated with more severe ANS dysregulation and increased need for mechanical ventilation (MV) up to 24h a day.14,15 Cases due to NPARMs are associated with a higher incidence of tumours and Hirschsprung disease.16
Ventilator dependence usually begins after birth (early-onset CCHS); although in some cases, hypoventilation remains undetected or manifests at later ages. Diagnosis is usually made one month after birth and in some cases at adult ages in adult carriers of mosaic or less severe mutations.17–20 There are other forms of primary central hypoventilation with late onset and no known associated mutation that differ from CCHS, such as rapid-onset obesity with hypoventilation, hypothalamic dysfunction and autonomic dysregulation (ROHHAD).21,22
Children with CCHS require optimised MV at home to survive and/or achieve optimal neurodevelopmental outcomes, therefore early diagnosis and continuous monitoring of their respiratory status are key to prevent complications and sequelae secondary to hypoxaemia and hypercapnia.15,23
This is a very rare disease of unknown prevalence, although the latter is estimated at 1 in 200000 live births.24 The European Central Hyperventilation Syndrome Consortium was constituted in 2010 with the purpose of optimising the management of these patients25 (www.ichsnetwork.eu/), and within this framework an online European registry of patients was created, that adheres to current regulations on safety, anonymity and confidentiality. At present, fourteen European countries, including Spain, participate in the registry. The objectives of this study were to: (a) learn the phenotypic and genotypic characteristics, clinical presentation, MV approaches used and outcomes of Spanish patients with a CCHS diagnosis included in the European registry, and (b) identify relevant aspects in the management of these patients that could be improved.
MethodsThe implementation of the European registry in Spain was authorised by the competent local Clinical Research Ethics Committee and announced through different scientific societies and the Spanish Association of CCHS patients and their families (www.sindromedeondine.es). Patient recruitment started in March 2012. Four families chose not to participate. The patients and/or legal guardians signed the informed consent form for participation. The Spanish clinicians in charge of these patients, one of whom is the Spanish registry coordinator, were given access to the online registry. Clinical data were entered by the participating clinicians and reviewed by the Spanish Registry coordinator. The data are updated periodically, and the last update took place in December 2015.
The following data were collected: date and country of birth, sex, ethnicity, pregnancy, delivery and neonatal period. Clinical manifestations at onset and during the course of disease (respiratory, gastrointestinal, neurologic, cardiovascular, ophthalmologic, endocrine, oncological, and other) and comorbidities. Diagnosis: date, tests performed, genetic testing, mutation found. Treatment: use of and changes in ventilatory support techniques; surgery. Length of hospital stay. Social factors: school attendance, educational attainment, initial employment. Data related to death.
We conducted a cross-sectional observational study. We have expressed the data using the mean, median, standard deviation and range of quantitative variables and the absolute and relative frequency of qualitative variables. When the data permitted, we conducted hypothesis tests: we compared the mean lengths of hospital stay in days by means of the Mann–Whitney U test and analysed the association between the frequency of comorbidities and the different mutations using linear regression. The statistical analysis was performed with SPSS version 15.
ResultsDuring the period under study (March 2012 to December 2015), data were entered and updated for 38 patients born between 1987 and 2013, with a mean age of 13.6±7.45 years (median, 11.35 months; range, 5 months–28.6 years). Thirteen patients (34.2%) were older than 18 years. Seventeen (44.7%) were male, and twenty one (55.3%) female. The regional origin of the patients was as follows: seven from Madrid, six from Andalusia, five from Castilla-La Mancha, five from Catalonia, three from Murcia, three from the Basque Country, two from arago, two from valencia one from the Cnaray Islands, one from Castilla León, one from Extremadura, one from the Balearic Islands, and one from Navarra. Twenty-two researchers from 19 Spanish hospitals participated in the study, and are named in the list of authors.
Three patients died (7.8%), all of them unexpectedly and out of the hospital, two during their sleep and one by accident. Their ages at the time of death were 5 months, 13 months and 15 years, respectively.
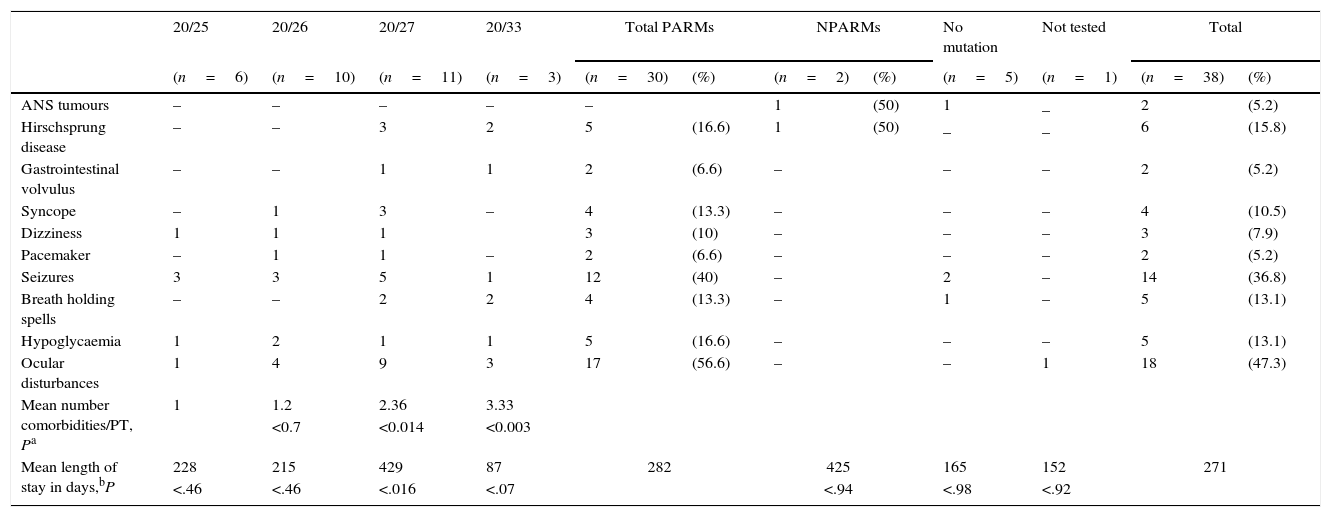
Genotypes, phenotypes and genotype-phenotype correlationPHOX2B screening was performed in 37 patients at the Spanish CCHS referral laboratory, Institute of Medical and Molecular Genetics (INGEMM), Hospital Universitario La Paz, Madrid. Mutations were detected in thirty-two patients (86.4%); thirty (93%) were PARMs and two (6.6%) were NPARMs (Table 1). Genotypes 20/25, 20/26 and 20/27 amounted to 84.3% of all detected mutations. No PHOX2B mutations were found in four children. Two received a diagnosis of ROHHAD syndrome based on their clinical presentation. In the first case hypoventilation onset was at age 10yrs, whereas the second case presented neonatal hypoventilation during non-REM sleep. For the remaining two patients, the result of the genetic test is unknown, for the first one and no genetic test was performed in the second case.
Genotype–phenotype correlations. Absolute frequency and percentage (in parentheses) of patients with different autonomic nervous system comorbidities in each group and subgroup of PHOX2B mutations (PARM [20/25, 20/26, 20/27, 20/33], NPARM).
| 20/25 | 20/26 | 20/27 | 20/33 | Total PARMs | NPARMs | No mutation | Not tested | Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n=6) | (n=10) | (n=11) | (n=3) | (n=30) | (%) | (n=2) | (%) | (n=5) | (n=1) | (n=38) | (%) | |
| ANS tumours | – | – | – | – | – | 1 | (50) | 1 | _ | 2 | (5.2) | |
| Hirschsprung disease | – | – | 3 | 2 | 5 | (16.6) | 1 | (50) | _ | _ | 6 | (15.8) |
| Gastrointestinal volvulus | – | – | 1 | 1 | 2 | (6.6) | – | – | – | 2 | (5.2) | |
| Syncope | – | 1 | 3 | – | 4 | (13.3) | – | – | – | 4 | (10.5) | |
| Dizziness | 1 | 1 | 1 | 3 | (10) | – | – | – | 3 | (7.9) | ||
| Pacemaker | – | 1 | 1 | – | 2 | (6.6) | – | – | – | 2 | (5.2) | |
| Seizures | 3 | 3 | 5 | 1 | 12 | (40) | – | 2 | – | 14 | (36.8) | |
| Breath holding spells | – | – | 2 | 2 | 4 | (13.3) | – | 1 | – | 5 | (13.1) | |
| Hypoglycaemia | 1 | 2 | 1 | 1 | 5 | (16.6) | – | – | – | 5 | (13.1) | |
| Ocular disturbances | 1 | 4 | 9 | 3 | 17 | (56.6) | – | – | 1 | 18 | (47.3) | |
| Mean number comorbidities/PT, Pa | 1 | 1.2 | 2.36 | 3.33 | ||||||||
| <0.7 | <0.014 | <0.003 | ||||||||||
| Mean length of stay in days,bP | 228 | 215 | 429 | 87 | 282 | 425 | 165 | 152 | 271 | |||
| <.46 | <.46 | <.016 | <.07 | <.94 | <.98 | <.92 | ||||||
ANS, autonomic nervous system; NPARM, nonpolyalanine repeat expansion mutation; PARM, polyalanine repeat expansion mutation; PT, patient.
Table 1 shows the referred comorbidities and their association with the different mutations; there was a high prevalence of ocular involvement (47.3%). Two patients underwent urgent surgery for volvulus of the gastrointestinal tract. Five patients (13.1%) had low glucose levels.
Due to the low number of registered patients presenting with NPARM mutations, we were not able to establish clear genotype/phenotype correlations differences between patients with PARM and patients with NPARM. However, structural disorders (ANS tumours, Hirschsprung disease) tended to be associated with NPARMs, while functional disorders (heart rate abnormalities, hypoglycaemia, etc.) were associated with PARMs (Table 1).
Within the group of patients with PARM, ANS disorders were more frequent and severe in patients with longer expansions (20/27 [P<.014] and 20/33 [P<.003]; Table 1).
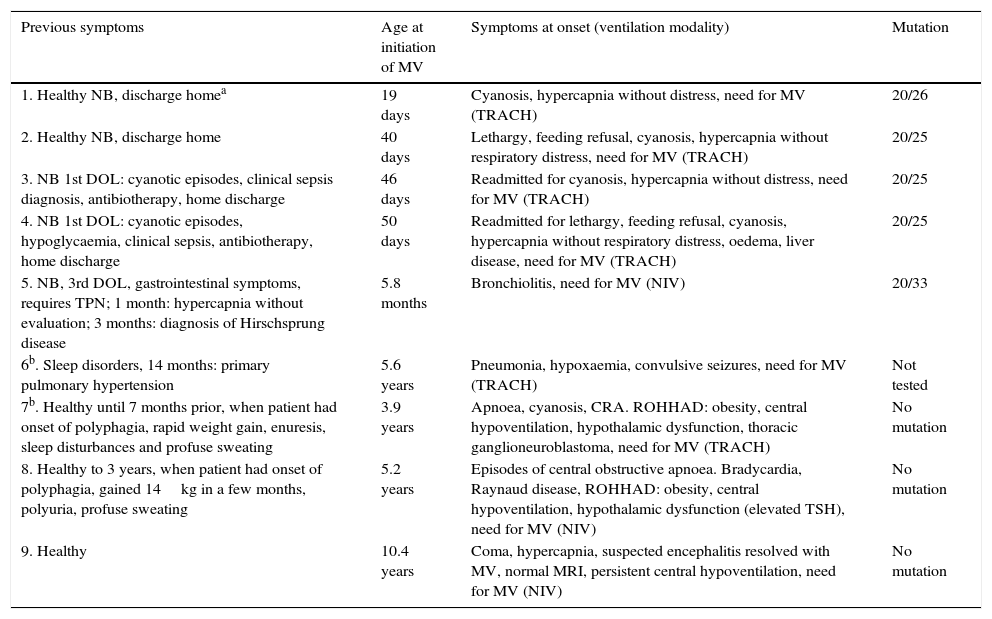
Initial clinical presentation formsThirty patients (79%) had typical clinical symptoms at onset and required MV starting in the neonatal period (29 from the first day of life, one later) without associated cardiorespiratory disease or any other clinical reason; a PHOX2B mutation was detected in 28 (93.3%) of them, no mutation was detected in one patient, and the results for the other patient are unknown. In eight patients (21%) (Table 2) the clinical presentation at onset was different and/or the need for MV occurred or was identified at later ages (late onset/delayed diagnosis). Genetic testing detected a PHOX2B mutation in four out of eight of these cases (50%).
Patients with late onset or delayed diagnosis of central hypoventilation syndrome.
| Previous symptoms | Age at initiation of MV | Symptoms at onset (ventilation modality) | Mutation |
|---|---|---|---|
| 1. Healthy NB, discharge homea | 19 days | Cyanosis, hypercapnia without distress, need for MV (TRACH) | 20/26 |
| 2. Healthy NB, discharge home | 40 days | Lethargy, feeding refusal, cyanosis, hypercapnia without respiratory distress, need for MV (TRACH) | 20/25 |
| 3. NB 1st DOL: cyanotic episodes, clinical sepsis diagnosis, antibiotherapy, home discharge | 46 days | Readmitted for cyanosis, hypercapnia without distress, need for MV (TRACH) | 20/25 |
| 4. NB 1st DOL: cyanotic episodes, hypoglycaemia, clinical sepsis, antibiotherapy, home discharge | 50 days | Readmitted for lethargy, feeding refusal, cyanosis, hypercapnia without respiratory distress, oedema, liver disease, need for MV (TRACH) | 20/25 |
| 5. NB, 3rd DOL, gastrointestinal symptoms, requires TPN; 1 month: hypercapnia without evaluation; 3 months: diagnosis of Hirschsprung disease | 5.8 months | Bronchiolitis, need for MV (NIV) | 20/33 |
| 6b. Sleep disorders, 14 months: primary pulmonary hypertension | 5.6 years | Pneumonia, hypoxaemia, convulsive seizures, need for MV (TRACH) | Not tested |
| 7b. Healthy until 7 months prior, when patient had onset of polyphagia, rapid weight gain, enuresis, sleep disturbances and profuse sweating | 3.9 years | Apnoea, cyanosis, CRA. ROHHAD: obesity, central hypoventilation, hypothalamic dysfunction, thoracic ganglioneuroblastoma, need for MV (TRACH) | No mutation |
| 8. Healthy to 3 years, when patient had onset of polyphagia, gained 14kg in a few months, polyuria, profuse sweating | 5.2 years | Episodes of central obstructive apnoea. Bradycardia, Raynaud disease, ROHHAD: obesity, central hypoventilation, hypothalamic dysfunction (elevated TSH), need for MV (NIV) | No mutation |
| 9. Healthy | 10.4 years | Coma, hypercapnia, suspected encephalitis resolved with MV, normal MRI, persistent central hypoventilation, need for MV (NIV) | No mutation |
CRA, cardiorespiratory arrest; DOL, days of life; MRI, magnetic resonance imaging; MV, mechanical ventilation; NIV, noninvasive mechanical ventilation with mask; NB, newborn; ROHHAD: rapid-onset obesity with hypoventilation, hypothalamic dysfunction and autonomic dysregulation; TPN, total parenteral nutrition; TRACH, tracheostomy.
Ventilatory support in the paediatric or neonatal intensive care unit: at onset, 35 patients (92.1%) received invasive MV following intubation, and 18 (47%) noninvasive ventilation (NIV), which was first used in year 2000. Fifteen children (39.5%) received both types.
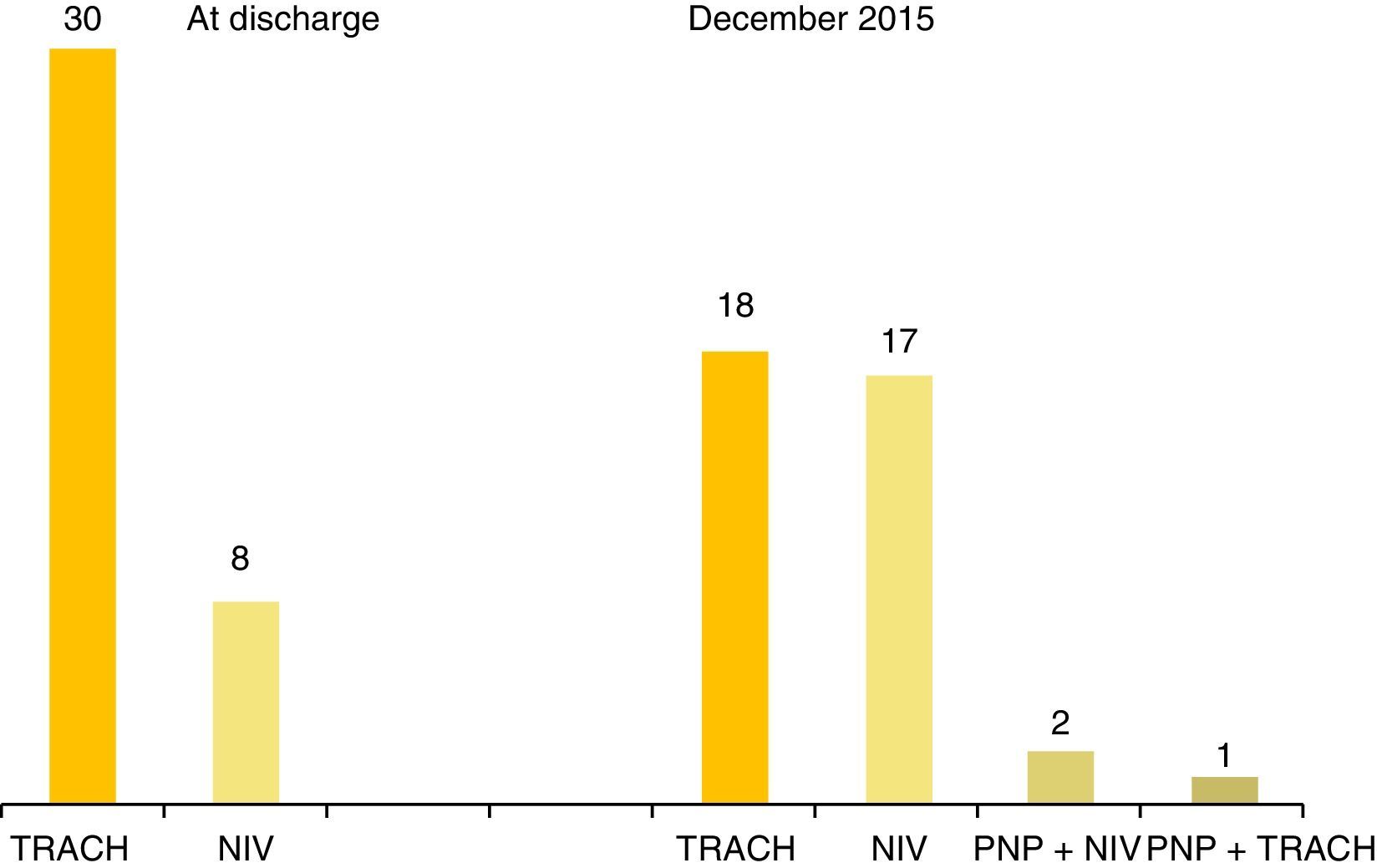
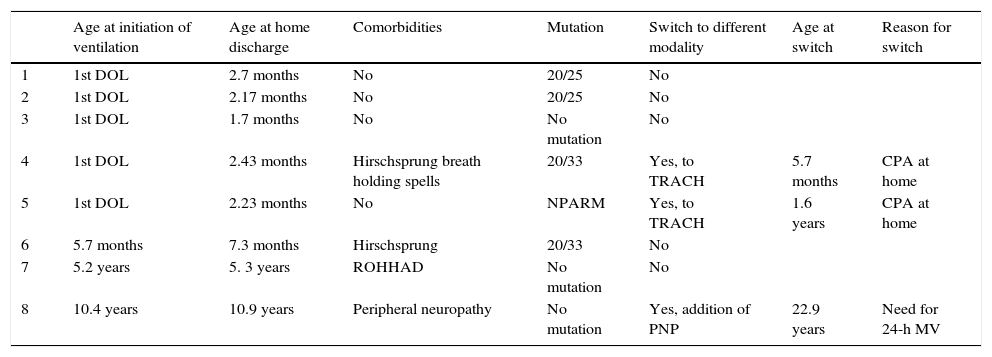
Ventilatory support at home: (a) via tracheostomy (TRACH): 32 patients (84.2%) received ventilation via TRACH; six (15.8%) never underwent tracheostomy; the mean age of surgery was 7.8 months±1.1 (median, 2.6 months; range, 24 days–5.75 years). Eleven patients were decannulated (34.3%); the mean age of decannulation and switching to NIV was 13.7 years±1.89 (median, 12.66 years; range, 6.12–20.5 years). Only one patient was aged less than 10 years at the time of decannulation. (b) Via mask: 19 patients (50%) received ventilation via mask at home; it was first used in 1998, a milestone that was published by the clinicians.26Table 3 shows the characteristics of the eight children ventilated via mask at the time of their first home discharge following diagnosis of CCHS: five were infants with neonatal onset, two of who had to switch to TRACH after experiencing cardiorespiratory arrest at home due to inefficient ventilation (genotype: 20/33, NPARM). (c) Phrenic nerve pacing (PNP): A PNP device was implanted in four children aged, 1.2, 8.8, 23, and 24yrs, respectively. The implantation of the PNP device failed in the youngest patients. The three remaining patients with PNP ventilation had reached adult age in December 2015, and were still needing MV around the clock (PNP during the day time and via TRACH (one patient) or via mask (two patients) at night time).
Patients who received noninvasive ventilation via mask as the first option and who remained under NIV after home discharge.
| Age at initiation of ventilation | Age at home discharge | Comorbidities | Mutation | Switch to different modality | Age at switch | Reason for switch | |
|---|---|---|---|---|---|---|---|
| 1 | 1st DOL | 2.7 months | No | 20/25 | No | ||
| 2 | 1st DOL | 2.17 months | No | 20/25 | No | ||
| 3 | 1st DOL | 1.7 months | No | No mutation | No | ||
| 4 | 1st DOL | 2.43 months | Hirschsprung breath holding spells | 20/33 | Yes, to TRACH | 5.7 months | CPA at home |
| 5 | 1st DOL | 2.23 months | No | NPARM | Yes, to TRACH | 1.6 years | CPA at home |
| 6 | 5.7 months | 7.3 months | Hirschsprung | 20/33 | No | ||
| 7 | 5.2 years | 5. 3 years | ROHHAD | No mutation | No | ||
| 8 | 10.4 years | 10.9 years | Peripheral neuropathy | No mutation | Yes, addition of PNP | 22.9 years | Need for 24-h MV |
CPA, cardiopulmonary arrest; DOL, days of life; MV, mechanical ventilation; NPARM: nonpolyalanine repeat expansion mutation; PNP, phrenic nerve pacing; ROHHAD: rapid-onset obesity with hypoventilation, hypothalamic dysfunction and autonomic dysregulation; TRACH, tracheostomy.
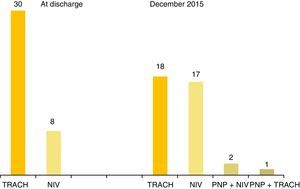
As of December 2015, twenty-two patients have used only one type of ventilatory support, thirteen patients two types, and three patients three types. None of the patients received negative-pressure ventilation. Fig. 1 shows the ventilatory support strategies used after the initial home discharge and in December 2015.
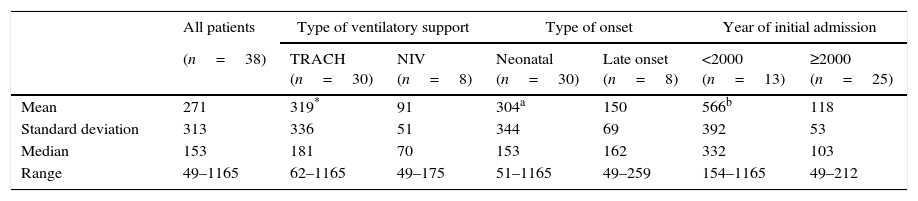
Lengths of hospital stay: Tables 1 and 4 present the length of the hospital stay preceding the use of MV at home. The length of stay was significantly longer in patients with 20/27 (P<.016), discharged with a TRACH (P<.004) or admitted before year 2000 (P<.000).
Length of hospital stay leading to initiation of mechanical ventilation at home. It was significantly longer in patients with tracheostomy and those admitted before year 2000.
| All patients | Type of ventilatory support | Type of onset | Year of initial admission | ||||
|---|---|---|---|---|---|---|---|
| (n=38) | TRACH (n=30) | NIV (n=8) | Neonatal (n=30) | Late onset (n=8) | <2000 (n=13) | ≥2000 (n=25) | |
| Mean | 271 | 319* | 91 | 304a | 150 | 566b | 118 |
| Standard deviation | 313 | 336 | 51 | 344 | 69 | 392 | 53 |
| Median | 153 | 181 | 70 | 153 | 162 | 332 | 103 |
| Range | 49–1165 | 62–1165 | 49–175 | 51–1165 | 49–259 | 154–1165 | 49–212 |
NIV, noninvasive ventilation with mask; TRACH, tracheostomy.
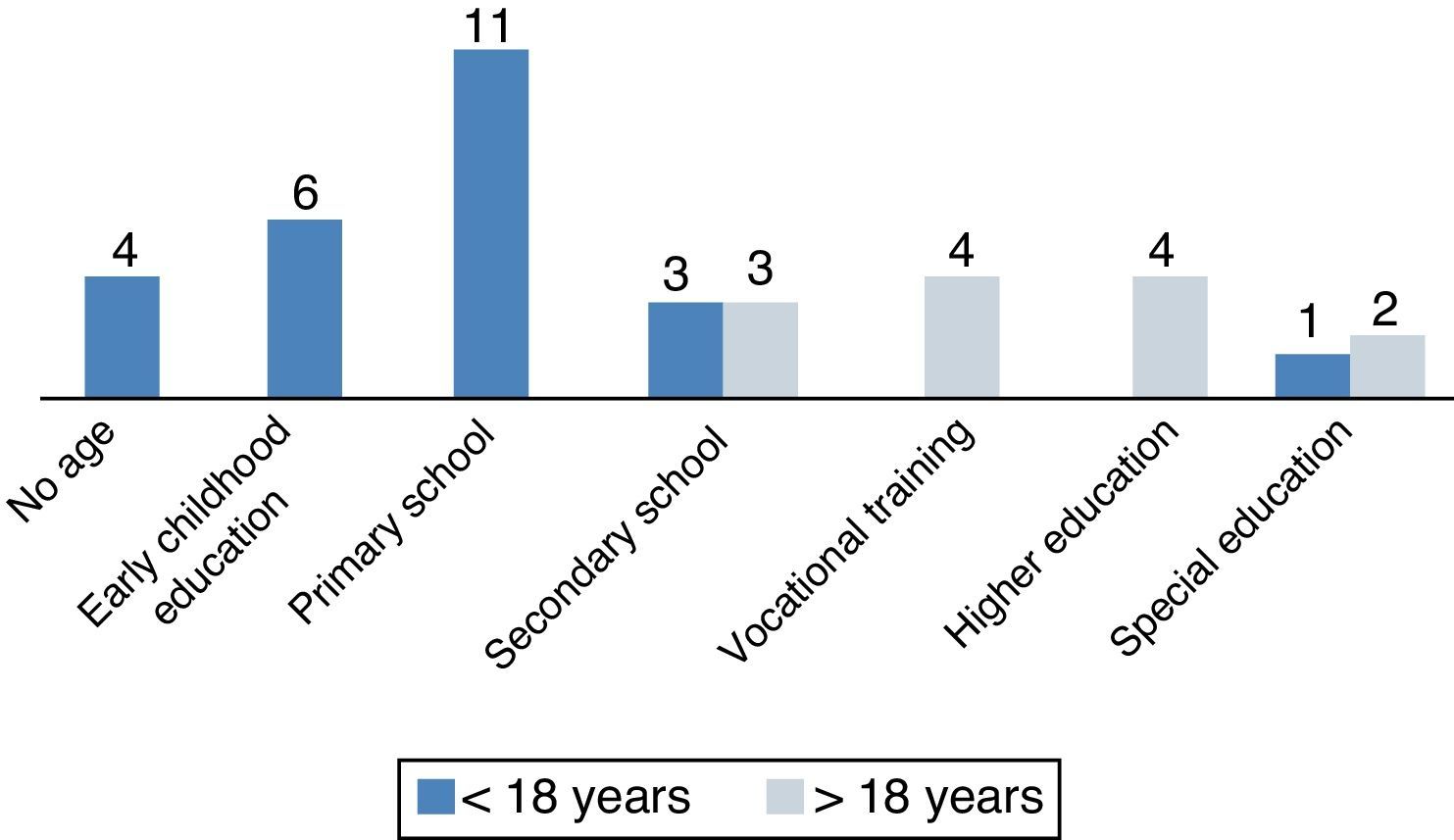
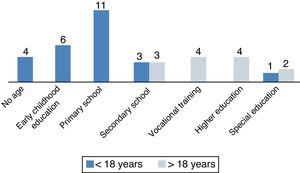
(a) School attendance: of the 34 school-aged patients, three (8.8%) attended special education programmes and 31 (91%) attended regular school, although ten of the latter (29.4%) required continuous special needs support. Fig. 2 shows the educational attainment of patients according to their ages. Four patients older than 18 years (30%) reached a higher education (university, 3; post-secondary vocational school, 1), (b) employment: four patients older than 18 years (30%) had begun remunerated employment.
DiscussionThis is the first comprehensive report on Spanish patients with CCHS. Due to its low prevalence and the absence of national reference centers in Spain for the management of CCHS patients, as recommended and practiced in other countries2 (France24, United States27), the implementation of the CCHS European Registry in Spain has required the efforts and collaboration of 19 Hospitals and 22 health care professionals, allowing the recruitment of 38 CCHS patients. The launching of the Spanish reference laboratory for the genetic diagnosis of CCHS is the first significant step taken towards this goal, which is an area for improvement.
One third of the registered Spanish CCHS patients are older than 18yrs as of December 2015. Their long survival being the consequence of the successfull at-home MV support and the continuous care and support provided by their families. Their entry into adulthood poses the challenge of the difficult transition of these chronically ill patients from paediatric care (i.e. with a close follow-up) to adult clinical care, i.e. to clinicians less experienced with this rare disease and therefore offering less protection to the patients. For this reason in some countries CCHS adult patients are followed by specialized reference centers in collaboration with clinical specialist.27 Living independently, becoming self-sufficient, living as a couple and entering working life may pose challenges to these young adults who sleep with a ventilator.
The neurocognitive development of patients with CCHS is influenced by disease-related factors, comorbidities and the permanent risk of hypoxaemia and hypercapnia, especially during sleep.28–30 Nevertheless, most Spanish patients have attended regular schools and four have attended higher education institutions, although one third required continued special needs support. For this reason, optimising the care of Spanish children with CCHS requires regular psychoeducational assessments and curriculum planning in order to promptly detect and manage any learning difficulties.
The type, frequency, and genotype-phenotype correlations identified in this study were consistent with those reported in previous case series.14 Although, as we mentioned above, the low number of patients with NPARMs in our study precluded comparative analyses; in contrast, in our cohort, ANS tumours were absent in patients with PARMs and Hirschsprung disease was more prevalent in patients with 20/33 and NPARM genotypes. Thus, genetic testing is essential for the diagnosis of the disease and in guiding the multidisciplinary management and regular followup according to genotype-based protocols, as recommended by the American Thoracic Society (ATS).2,15
As for the comorbidities, five patients (13%) had hypoglycaemia, as compared to 8% in the series published by Vanderlaan et al.8; this complication has been rarely reported, and even gone unmentioned in publications by experts2; yet it is of great clinical relevance, as hypoglycaemia has a considerable impact in the daily life of affected children and their families, both due to its symptoms and to the measures required to control it, which are not easy to achieve.31 Two patients (genotypes 20/27, 20/33) required urgent laparotomy due to gastrointestinal volvulus, a complication which has not been previously reported by other authors, although it may have been included in the broader category of intestinal motility disorders. Ophthalmological problems are very frequent overall, and were found in nearly half of the Spanish patients, as happened in the series published by Vanderlaan et al.8 Patwari et al5 described an impaired pupillary response to light. Therefore, regular ophthalmologic evaluations including pupillometry are needed to prevent potential negative repercussions of visual abnormalities on learning and development.
Symptoms of central hypoventilation may go unnoticed or be misinterpreted leading to delayed diagnosis, especially in patients with milder mutations, as happened in three of our patients with the 20/25 genotype, although it also happened in the case of infant with a 20/33 genotype who received an initial diagnosis of Hirschsprung disease. Delays in diagnosis result in chronic hypoxia and hypercapnia, which impair neurodevelopment20; their prevention is an area for improvement that calls for maintaining a high level of surveillance in patients with unexplained clinical findings such as episodes of cyanosis, lethargy, pulmonary hypertension, need for MV due to mild infections or after anaesthesia, seizures or neurodevelopmental delay.
The choice of ventilatory support modality in each patient and at each life stage is very important for the optimisation of care. Ventilation via TRACH is the most frequently used technique in Spanish children with CCHS in the first decade of life, consistent with previous reports in the literature.8 Different authors and the ATS recommend ventilation by TRACH during the early years of life due to its safety and in order to guarantee optimal neurodevelopmental outcomes, avoiding switching to NIV before age 6–8 years.15 However, this is subject to controversy, as there are no studies analysing the impact of the choice of ventilatory support modality on neurodevelopment. Costa Orvay et al.32 described the failure of NIV in two infants with CCHS in contrast to the experience of other authors33; in the series published by Vanderlaan et al., 14.3% of 196 patients never received ventilation via TRACH,8 a percentage similar to that found in our study (15.8%). We found that eight patients had been managed from the beginning and home discharged with mask ventilation and without TRACH, five of whom had onset during the neonatal period: the two with the most severe phenotypes (20/33, NPARM) had to switch to ventilation by TRACH after experiencing cardiorespiratory arrest at home, while the other three, who had milder mutations (20/25, 20/25, no mutation), continued with mask ventilation. A factor to consider in addition to genotype severity when NIV is used in younger children is the negative impact that the interface may have on facial development in the long term,34,35 which we did not analyse in this study. Given all of the above, it is advisable to use TRACH as the initial modality in children with early-onset CCHS, considering NIV as the alternative initial modality for very specific patients, such as babies with milder phenotypes associated with milder hypoventilation and no comorbidities, or patients with late onset. The use of NIV should always be combined with close monitoring and preventive measures aimed to avoid facial deformities (alternating interfaces, use of full-face mask or negative pressure ventilation).36,37
Phrenic nerve pacing associated with other modalities was only used by three Spanish patients, all of whom were dependent on 24-h a day MV, consistent with the reports of other authors.38 However, PNP was used only in adolescents or adults only ventilated during sleep8,27 who have been decannulated and no longer use a mask. The drawbacks of PNP are the surgery needed for implanting the device and the technical or infectious complications.39 A new device that directly stimulates the diaphragm could improve this approach, although there is still little evidence on its use.40
In our patients, the transition from TRACH to NIV took place at a mean age of 13.7 years, which is double the minimum age recommended for safety (6–8 years)15; only one child transitioned before age 10 years, and various factors that were not analysed in the study influenced the case-by-case decision of the timing for the switch: advantages/disadvantages, attitudes and wishes of patients and parents, or the medical team experience.
The main limitation of this study is that the Registry has not recruited every Spanish patient with CCHS because some families declined to participate and there are probably additional patients who have not been diagnosed or remain unknown. Using the data of known patients and the demographic data published by the Instituto Nacional de Estadística of Spain (National Institute of Statistics, http://www.ine.es/inebmenu/mnu_dinamicapob.htm), we estimate a CCHS prevalence of 3.5 per 1000000 live births; however, if we take the prevalence in France as a reference,24 approximately two children would be born with CCHS in Spain every year, and there would be 17 individuals with CCHS that have yet to be identified. We would need to increase our collaborative efforts in order to identify them.
In short, the introduction of the European Registry of CCHS patients in Spain has allowed us to identify relevant factors for the purpose of improving their clinical management: the absence of reference hospitals in Spain, the importance of genetic testing and education strategies, and some limitations of different ventilatory support modalities.
FundingThis study was partially funded by grant FIS08/90233 (Angel Campos-Barros). The design, development and maintenance of the European registry have been funded by the Consumer, Health and Food Executive Agency of the European Commission.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the patients with CCHS and their families, as well as the European Central Hypoventilation Syndrome Consortium.
Ana Llorente de la Fuente: Cuidados Intensivos Pediátricos, Hospital Doce de Octubre, Madrid, Spain
Arturo Hernández González: Cuidados Intensivos Pediátricos, Hospital Puerta del Mar, Cádiz, Spain
Amaya Bustinza Arriortua: Cuidados Intensivos Pediátricos, Hospital Gregorio Marañón, Madrid, Spain
Jesús de la Cruz Moreno: Pediatría, Hospital Universitario Materno Infantil, Jaén, Spain
Martí Pons Odena: Cuidados Intensivos Pediátricos, Hospital Sant Joan de Déu, Barcelona, Spain
Purificación Ventura Faci: Neonatología, Hospital Lozano Blesa, Zaragoza, Spain
Laura Rubio Ortega: Hospitalización a Domicilio Pediátrica, Hospital General Universitario, Alicante, Spain
Estela Pérez Ruiz: Neumología Infantil, Hospital Carlos Haya, Málaga, Spain
Antonio Aguilar Fernández: Neumología Pediátrica, Hospital Materno Infantil, Las Palmas, Spain
Amaya Pérez Ocón: Cuidados Intensivos Pediátricos, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain
Borja Osona: Neumología Pediátrica, Hospital Son Espases, Palma de Mallorca, Islas Baleares, Spain
Isabel Delgado Pecellin: Neumología Pediátrica, Hospital Virgen del Rocío, Sevilla, Spain
Ignacio Arroyo Carrera: Neonatología, Hospital San Pedro de Alcántara, Cáceres, Spain
Javier Sayas Catalán: Neumología, Hospital Doce de Octubre, Madrid, Spain
Elvira González Salas: Cuidados Intensivos Pediátricos, Hospital Universitario de Salamanca, Salamanca, Spain
Carlos Martin de Vicente: Neumología Pediátrica, Hospital Miguel Servet, Zaragoza, Spain
Please cite this article as: García Teresa MA, Porto Abal R, Rodríguez Torres S, García Urabayen D, García Martínez S, Trang H, et al. Pacientes españoles con síndrome de hipoventilación central incluidos en el Registro europeo. Datos del 2015. An Pediatr (Barc). 2017;86:255–263.