Since its approval by the European Medicines Agency, a great number of patients born small for gestational date have received recombinant growth hormone treatment in Spain. The aim of this study is to analyse its outcome in the setting of ordinary clinical practice.
MethodsInformation was gathered from the registers of the assessment boards that authorise all growth hormone treatments prescribed in public hospitals in six autonomic communities (regions).
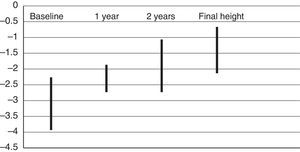
ResultsValid data from 974 patients was obtained. All of them complied with criteria established by the European Medicines Agency. Patients in the sample were smaller in length than weight at birth, with their median target height being below 1 standard deviation (SD), and 23% of them had been delivered prematurely. Treatment was started at 7.2±2.8 years (mean±SD). The mean patient height at start was −3.1±0.8 SD. They gained 0.7±0.2 SD in the first year, and 1.2±0.8 SD after two years. Final height was attained by 8% of the sample, reaching −1.4±0.7 SD.
ConclusionsThese results are similar to other Spanish and international published studies, and are representative of the current practice in Spain.
Despite treatment being started at a late age, adequate growth is observed in the short term and in the final height. Up to a 24% of patients show a poor response in the first year.
Desde su aprobación por la Agencia Europea del Medicamento, el tratamiento con hormona de crecimiento recombinante ha sido empleado en un gran número de pacientes nacidos pequeños para la edad gestacional en España. El propósito de este estudio es conocer objetivamente los resultados del mismo en la práctica habitual.
MétodosSe ha recogido información procedente de los registros existentes en los comités asesores que autorizan dichos tratamientos en los hospitales públicos de 6 comunidades autónomas.
ResultadosSe han obtenido datos válidos de 974 pacientes. Todos ellos cumplían los criterios exigidos por la Agencia Europea del Medicamento. Los pacientes que recibieron el tratamiento se caracterizaron por tener la longitud al nacer más afectada que el peso, talla diana inferior a –1 desviación estándar (DE) y un 23% con antecedentes de prematuridad. La talla al iniciar el tratamiento fue de −3,1±0,8 DE (media±DE) y la edad de comienzo 7,2±2,8 años. La ganancia de talla en el primer año fue de 0,7±0,2 DE, y de 1,2±0,8 DE hasta los 2 años. La talla final, alcanzada por un 8% de pacientes, fue de –1,4±0,7 DE.
ConclusionesLos resultados concuerdan con las series nacionales e internacionales publicadas y son representativos de la práctica habitual en nuestro país.
Se constata un inicio tardío del tratamiento, observándose, sin embargo, un adecuado crecimiento, tanto a corto plazo como en la talla final. En el primer año se identifica un 24% de pacientes con respuesta deficiente.
The term small for gestational age comprehends all newborns whose birth weight or birth crown-heel length is 2 sd below the mean for their sex and gestational age based on a reference population.1 Since 2003, when the European Medicines Agency approved the use of recombinant human growth hormone (rhGH) for the treatment of children small for gestational age (SGA) or with intrauterine growth restriction (IUGR) without postnatal catchup growth, a large number of patients have received this treatment in Spain. However, we do not know the exact number of patients, their geographical distribution, the age at treatment initiation, the clinical response and the potential adverse effects that have been observed. Much of this information can be found in the registers of the advisory committees that authorise rhGH treatment in the different autonomous communities (regions) of Spain. In order to obtain an objective understanding of the characteristics of the use of rhGH in children born SGA in Spain, we collected the data from these registers in several autonomous communities as a preliminary phase for a nationwide study.
After analysing the data and correcting potential errors in the calculated values, we compared the results with those of domestic and international studies on the use of rhGH for the treatment of SGA children.
MethodsWe collected all the data contained in the registers of the advisory committees that regulate the distribution of rhGH by the Department of Public Health of the different autonomous communities on the patients in whom treatment was authorised, with the exception of confidential personal information. The collected data included parental heights, gestational age, birth weight and birth length. We also recorded the age, weight, height, pubertal stage of development and growth velocity of patients at the time of treatment initiation and in subsequent checkups, as well as the prescribed doses of rhGH and the final height achieved by those that completed treatment during the period under study. We also recorded the age at menarche in female patients.
We assessed auxologic measurements using the charts of the Estudio Colaborativo Español 20082 and pubertal development by means of Tanner scale.3 We summarised the data as means and standard deviations (SDs). We used the IBM SPSS 19.0 software for Windows for the statistical analysis. We conducted two-tailed Student's t tests and paired-sample t tests. We defined statistical significance for all the analysed variables as a p-value of less than 0.05 for a 95% confidence interval.
ResultsWe analysed the treatments (number) registered from 2004 to 2014 in the autonomous communities of Aragón (76), Basque Country (87), Navarra (56), Catalonia (525), Cantabria (69) and Galicia (161). We obtained valid data for a total of 974 patients (48% male). These patients amounted to approximately 21% of the total rhGH treatments authorised in those autonomous communities. Five percent of the patients were born from multiple gestations and 23% born preterm, and the gestational age for the overall sample was 38±3 weeks.
The mean birth weight was −2±1 SD and the mean birth length was −2.6±0.9 SD.
At birth, the length of 81% of the patients was at least 2 SDs below the mean and the weight of 54% at least 2 SDs below the mean, and both were at least 2 SDs below the mean in 42% of the patients.
In 27% of the patients, the target heights ([father's height+mother's height±13]/2) were at least 2 SDs below the mean. The mean target height was −1.5±1 SD.
Patients started receiving treatment with rhGH at an age of 7.2±2.8 years. During the period under study, the age at treatment initiation did not change significantly in the more recent patients.
The height at treatment initiation was −3.1±0.8 SD, and the baseline growth velocity was −1.4±1.5 SD. The stage of pubertal development at initiation of treatment was Tanner I in 89%, Tanner II in 7% and Tanner III in 2%.
The initial dose of rhGH was 35±6μg/kg/day, and we found no significant changes at follow up or depending of the year in which treatment was initiated; the initial dose was also independent of age and stage of pubertal development at initiation. All patients received uninterrupted treatment.
The height gains associated with treatment corresponded to 0.7±0.2 SDs in the first year, and 1.2±0.8 SDs in the first two years. Approximately 30% of patients achieved height z-scores greater than −2 SD in the first year of treatment, and another 60% of patients achieved them in the second year (Fig. 1). In the fifth year of treatment, heights had increased by 2±1.9 SDs compared to baseline. Growth velocity increased by 3.9±2.3 SDs compared to the year before treatment initiation. We did not find statistically significant differences in treatment response based on gestational age.
Fourteen percent of patients exhibited a response in the first year of treatment that was more than 2 SDs below the mean for the sample, corresponding to height gains of less than 0.3 SD, which can serve as the threshold for defining nonresponders. Up to 24% had height gains of less than 0.5 SD and 55% had growth velocity increases of less than 3cm/year.
The response in the first year of treatment was negatively correlated with initial height (r=−0.40) and the age at initiation of treatment (r=−0.36), and positively correlated with the rhGH dose (r=0.24), associations that were statistically significant (P<.05).
Eight percent of the patients reached their final heights during the follow-up, corresponding to a mean of −1.4±0.7 SD, very similar to the target height, with a difference of −0.1±1 SD.
The age at menarche, available for 13% of female patients, was 13.3±2 years.
DiscussionSeveral studies conducted abroad on the use of rhGH in SGA children with no postnatal catchup growth support its efficacy and safety.4,5 Following its approval by EMA, the Spanish Ministry of Health introduced its indication under the category of intrauterine growth restriction. In the past few years, several publications have reported the outcomes of treatment in different case series that included patients from different hospitals.6–10
However, until now we had no data on the number of SGA patients treated with rhGH and their proportion compared to other indications for the use of rhGH, their geographical distribution, and the details concerning their followup. Our study analysed data for all SGA children treated with rhGH in a specific geographical area that comprised six autonomous communities in the north of Spain, a preliminary phase in a nationwide study, and included 974 patients that received this treatment in the 2004–2014 period. Our study has the limitations imposed by the source of the data, as the registers do not collect exhaustive information on obstetric factors, comorbidities, side effects or adherence to treatment. However, given the considerable number of patients included and the absence of selection bias (they were treated in different hospitals and received rhGH made by different manufacturers), our study is representative of the use of rhGH for the treatment of SGA children (IUGR) in Spain.
Our results reveal a characteristic profile in the patients in whom treatment with rhGH is authorised: target height below the mean, smaller birth lengths compared to birth weights, and in nearly 25%, preterm. This profile could be used to identify SGA children whose growth should be monitored more closely in the early years of life. Other series have also described target heights of approximately 1 SD below the mean,11,12 but in our series 27% of the target heights were at least 2 SDs below the mean, suggesting a marked genetic component in the short stature of this group of patients.
Children born preterm, who amount to 5% to 9% of births in Europe,13 were disproportionally represented in our sample, amounting to 23% of the patients, although this proportion was similar to the one found by another study conducted in Spain, the DATAC study, in which 27% of patients had been born preterm.10 The response to treatment of children born preterm was comparable to that of children born at term.14
Although treatment is approved by the EMA starting at age 4 years, the mean age at initiation in our patients was 7.2 years, a delay that did not improve during the period under study. Other studies conducted in Spain reported similar ages.6,7,9,10 We hypothesise that the reason for this is that the practise of referring all SGA children that do not experience catchup growth in the early years to paediatric endocrinology services is not yet widespread.
The response to rhGH, expressed as an increase in height SDs, is greater the younger the age at initiation, a finding that was confirmed in our study with a strong negative correlation between age and height gain in the first year of treatment. The findings of other series are similar.8,11,12 An adequate follow-up of SGA children from birth would allow initiation of treatment and the normalisation of stature at earlier ages.15
We found that patients had uniformly received a mean dose of 35μg/kg/day, which did not change during the follow-up nor based on age or the time to initiation of treatment. Although the dose adjustment during puberty remains controversial,16,17 we also found no evidence of dose adjustment based on the stage of pubertal development. On the other hand, different doses of rhGH do not lead to significantly different outcomes in the long term.18,19
Our study corroborated that treatment with rhGH in children SGA is generally efficacious, both in the early stages and in patients reaching their final heights, with the latter achieving values within the range of the target height, a goal that has not always been achieved in previous studies.18,20–22
However, there was variability in the response to treatment, with 14% of nonrespondents, which in our study was defined as a height increase of less than 0.3 SD in the first year, and up to 24% if we were to apply the criteria of height gain less than 0.5 SD.23 Some studies have defined inadequate response as an increase of less than 3cm in the growth velocity.24 Based on this criterion, 55% of the patients in our sample would be categorised as nonrespondents. In any case, the mean response values in our patients are consistent with those reported in other studies,25 both in Spain (Table 1) and internationally (Table 2). Higher doses were used in the series of Carrascosa et al.,7 Argente et al.8 and Boguszewski et al.,26 and the last two included younger patients. The meta-analysis conducted by Maiorana and Cianfarani,27 which included several European studies, found that patients failed to achieve their target heights, despite the use of higher doses compared to our study, possibly due to initiation of treatment at older ages. The largest series published to date, in the context of the KIGS study and funded by Pfizer®, with 900 patients and a mean dose of 40μg/kg/day, found a height gain of 0.7 SD in the first year, as did our study, but had final heights around −2 SD.11 In the series of Ridder et al.,28 the final height was far below the target height, despite the use of doses of up to 67μg/kg/day. The height catchup observed in our sample was similar to that observed in patients treated for growth hormone deficiency.25,29
Studies conducted in Spain.
| STUDY | Luzuriaga Tomás et al.,6 2003 | Carrascosa et al.,7 2006 | Argente et al.,8 2007 | López Siguero et al.,9 2013 |
|---|---|---|---|---|
| Number of patients | 42 | 68 | 76 | 147 |
| Target heighta (z-score) | −2±0.6 | −1.2±0.9 | −1.0±0.5 | −0.9±0.7 |
| Age at initiation (years)a | 8.1±2.3 | 7.1±2.4 | 4.3 | 7.4±2.8 |
| Baseline heighta (z-score) | −2.5±0.3 | −3.3±0.6 | −3.1±0.3 | −3.0±0.6 |
| Height at 1 yeara (z-score) | −1.7±0.4 | −2.1±0.7 | −1.6 | −2.3±0.7 |
| Δ Height in 1st year (z-score) | 0.8 | 1.2 | 1.5 | 0.7 |
| Dose (μg/kg/day) | 65 | 66 | 60 | 35 |
International studies.
| Study | KIGS,11 2007 | De Ridder et al.,28 2008 | Kriström et al.,30 2009 | Boguszewski et al.,26 2014 | Maiorana and Cianfarani27 | Current series |
|---|---|---|---|---|---|---|
| Number of patients | 900 | 150 | 367 | 156 | 391 | 974 |
| Target height (z-score) | −1.1 | −0.7 | −0.7 | −0.9 | −1.1 | −1.5 |
| Age at initiation (years) | 7.1 | 7.5 | 8.3 | 3.3 | 9 | 7.2 |
| Baseline height (z-score) | −3.4 | −3.1 | −2.9 | −3.9 | −2.9 | −3.1 |
| Height at 1 year (z-score) | −2.7 | −2.2 | −3 | −2.4 | ||
| Δ Height in 1st year (z-score) | 0.7 | 0.7 | 0.9 | 0.7 | ||
| Dose (μg/kg/day) | 40 | 33/67 | 33 | 50 | 33–67 | 35 |
| Final height (z-score) | −2.1 | −1.4 | −1.5 | −1.4 |
Values are expressed as means.
The response observed in the first year of treatment is a predictor of final height.30,31 In our sample, the determinants of the initial response were the age at initiation of treatment, the initial height and the dose of rhGH, which was consistent with other studies.28,31
Although we did not have detailed information on the onset of puberty, the age at menarche in 13% of the female patients was 13 years. While treatment with rhGH does not seem to affect the onset and development of puberty in general,32 a longer followup period is required for confirmation. The SGA population enters puberty at an age close to the mean for the general population, although girls with low birth weights that experience rapid weight and height catchup tend to reach puberty at earlier ages33 and may present with pronounced adrenarche and hyperandrogenism associated with hyperinsulinism.34
The analysis of the data obtained from the official registers of the autonomous communities did not allow us to draw conclusions regarding the safety of treatment with rhGH in SGA patients. However, the evidence in the existing literature consistently suggests that there are no significant risks—at least in the intermediate term—with frequent elevation of blood insulin levels that resolves spontaneously.1,4,5,35
Conclusions- (1)
We present a study based on a population of SGA children managed with rhGH in Spain, with no bias from the use of a specific brand of recombinant hormone, treated in several different hospitals following the criteria of the EMA with the approval the advisory committees of the specific autonomous communities. It includes cases from the initial authorisation for its use in IUGR/SGA to the recent past.
- (2)
The sample of 974 patients was the largest studied to date, and comparable to those of international studies that included patients from different countries. We believe that this study is representative of the use of rhGH for the treatment of IUGR/SGA in Spain.
- (3)
We found that most patients responded well to treatment from the first year, achieving final heights that were close to the genetic potential or target height. Twenty-four percent of the patients responded poorly, with height gains of less than 0.5 SD in the first year.
- (4)
The information pertaining to adverse effects and pubertal development was not recorded in the database with adequate accuracy, and should be investigated with different methods.
- (5)
The data underscore the importance of starting treatment with rhGH early with the purpose of achieving normal heights in children born SGA. Small for gestational age children whose length was more affected than weight at birth, whose parents have short statures or born preterm should be monitored closely in the first three years of life to initiate treatment promptly if they do not exhibit spontaneous catch-up growth.
The authors have no conflicts of interest to declare
We thank the prescribing paediatricians in Aragón, Cantabria, Catalonia, Basque Country, Galicia and Navarra, as well as the advisory boards and committees on growth hormone of those autonomous communities, for providing the data analysed in the study.
Please cite this article as: Rial Rodríguez JM, de Arriba Muñoz A, Bosch Muñoz J, Cabanas Rodríguez P, Cañete Estrada R, Díez López I, et al. Tratamiento con hormona de crecimiento en pequeños para la edad gestacional en España. An Pediatr (Barc). 2017;86:249–254.