Small for gestational age (SGA) children without catch-up growth can benefit from treatment with growth hormone (rhGH). However, they should be monitored very closely because they are at increased risk of metabolic syndrome.
Material and methodA group of 28 SGA children with a mean age of 8.79 years and undergoing treatment with rhGH were selected for evaluation. Over the course of 4 years, an annual evaluation was performed on the anthropometric variables (weight, height, body mass index [BMI], growth rate, blood pressure and waist perimeter), metabolic risk variables (glycaemia, glycosylated haemoglobin, cholesterol ratio, insulinaemia, insulin-like growth factor 1[IGF1], IGF binding protein-3 [IGFBP-3], IGF1/IGFBP3 ratio, and HOMA index), and body composition variables.
ResultsTreatment with rhGH was associated with a significant increase in height (−2.76±.11 SD to −1.53±.17 SD, P=.000), weight (−1.50±.09 SD to −1.21±.13 SD; P=.016), and growth rate (−1.43±.35 SD to .41±.41 SD; P=.009), without a corresponding change in the BMI. Insulinaemia (9.33±1.93mU/ml to 16.55±1.72mU/ml; P=.044) and the HOMA index (3.63±.76 to 6.43±.67; P=.042) increased, approaching insulin resistance levels. No changes were observed in the lipid profile. Body composition changes were observed, with a significant increase in lean mass (73.19±1.26 to 78.74±1.31; P=.037), and a reduction of fat mass (26.81±1.26 to 21.26±1.31; P=.021).
ConclusionTreatment with rhGH is effective for improving anthropometric variables in SGA patients who have not experienced a catch-up growth. It also produces changes in body composition, which may lead to a reduction in risk of metabolic syndrome. However, some insulin resistance was observed. It is important to follow up this patient group in order to find out whether these changes persist into adulthood.
Los niños pequeños para la edad gestacional (PEG) sin crecimiento recuperador pueden beneficiarse del tratamiento con hormona de crecimiento (rhGH). Sin embargo, deben ser monitorizados de forma muy estrecha ya que son población de riesgo metabólico.
Material y métodosSe han incluido 28 niños PEG, con una media de edad de 8,79 años, sin crecimiento recuperador, tratados con rhGH. Hemos evaluado las modificaciones producidas en la antropometría, variables de riesgo metabólico y composición corporal durante 4 años de tratamiento.
ResultadosEl tratamiento con rhGH se acompañó de un aumento de talla (−2,76±0,11 DE hasta −1,53±0,17 DE; p=0,000), peso (−1,50±0,09 DE hasta −1,21±0,13 DE; p=0,016) y velocidad de crecimiento (−1,43±0,35 DE hasta 0,41±0,41 DE; p=0,009), sin producir modificaciones en el índice de masa corporal (IMC). Se han visto aumentos significativos de la insulinemia (9,33±1,93mU/ml hasta 16,55±1,72mU/ml; p=0,044) y del índice HOMA (3,63±0,76 hasta 6,43±0,67; p=0,042), sin producirse modificaciones en el perfil lipídico. En el estudio de composición corporal se ha comprobado un aumento significativo de la masa magra (73,19±1,26 hasta 78,74±1,31; p=0,037) con una disminución de la masa grasa (26,81±1,26 hasta 21,26±1,31; p=0,021).
ConclusiónEl tratamiento con rhGH se ha acompañado de una ganancia en la talla sin producir alteraciones en el IMC. Asimismo, se han observado cambios en la composición corporal, con un aumento de la proporción de masa magra a expensas de una disminución de la de masa grasa, que podrían conducir a un descenso del riesgo metabólico de estos pacientes. Sin embargo, se ha detectado cierta resistencia insulínica. Es importante continuar el seguimiento de estos niños para determinar las posibles repercusiones en la edad adulta.
It is estimated that between 3% and 5% of newborns are small for gestational age (SGA),1 with a progressive increase in frequency in the past decade.2 Small for gestational age is defined as birth weight and/or length at least two standard deviations (SDs) below the mean for gestational age, established based on data from the reference population.3
The causes that can lead to SGA can be grouped into foetal, maternal, placental and environmental factors, although the aetiology of intrauterine growth restriction cannot be determined in one third of the cases.2
Small for gestational age children are at higher risk of abnormalities in body composition, disorders of puberty, neurodevelopmental delay and metabolic syndromes, and have a higher prevalence of risk factors for cardiovascular disease.
Eighty to ninety percent of SGA children will experience spontaneous catch-up growth and achieve a normal height at around age 2 years,4 and may eventually reach their genetic potential.3–5 Still, ten to twenty percent of these children will not have catch-up growth, and the cause for this is unknown. After age 4 years, spontaneous catchup becomes very unlikely.6,7 In 2003, the European Agency for the Evaluation of Medical Products (EMEA) and the Food and Drug Administration (FDA) approved the use of recombinant human growth hormone (rhGH) for the treatment of these children.
Growth hormone causes an increase in growth rate that allows these children to achieve a normal final height.8,9 In addition to its effects on linear growth, rhGH is an anabolic hormone that affects the metabolism of fats and carbohydrates,10 which leads to changes in body composition.11
The aim of our study was to analyse the changes in anthropometric, metabolic risk and body composition variables in a group of SGA children before initiation of treatment with rhGH and during the four-year followup.
MethodsStudy design and participantsWe conducted a prospective longitudinal study in a cohort of 28 children born in the Hospital Clínico Universitario de Zaragoza between 1995 and 2009 with a diagnosis of SGA. Participants were included consecutively. There was no control group. The follow-up period was four years, and we compared the sample data for different points in time. Seventy-one percent of the sample completed the four-year followup, while the remaining 29% patients were in earlier stages of treatment by the end of the study, as they had been included at later times.
The patients included in the study fulfilled the criteria required by the Advisory Committee on Growth Hormone of the Ministry of Health, Social Services and Equality for its use in SGA children:
- –
Birth length and/or weight at least 2 SDs below the reference standards.12
- –
From age 4 years, stature at least 2.5 SDs below the reference standard.13
- –
Lack of catch-up growth by age 4 years.
- –
Ruling out of other medical problems or treatments as the cause of the growth disorder.
The exclusion criteria were the following:
- –
Patients that did not meet the auxologic criteria for inclusion.
- –
Presence of certain syndromes that may not benefit from the therapy.
- –
Diabetes mellitus or other significant disorder of carbohydrate metabolism.
We collected the neonatal data of participants: birth weight and length (z-score)12 and gestational age in weeks. We calculated the genetic potential length (SD).
We collected data for the following variables before initiating treatment and in the four years of followup:
Clinical and anthropometric variables- –
Anthropometric variables: weight,13 length,13 body mass index (BMI), and growth velocity (GV), all in SDs.
- –
Clinical variables: systolic and diastolic blood pressure (SBP, DBP) measured with an automated blood pressure monitor and choosing the cuff size based on the arm circumference. Blood pressure in mmHg and its z-score based on reference charts.14 Waist circumference in cm and its z-score.15
Plasma glucose (mg/dL), percent glycosilated haemoglobin (Hb), triglycerides (TG) in mg/dL (TG), cholesterol ratio (total cholesterol [TC]/HDL), IGF1 (ng/mL), IGF1BP3 (μg/mL), IGF1/IGF1BP3 ratio, plasma insulin (mIU/mL) and the homeostatic model assessment (HOMA) index (glucose [mg/dL]×insulin [mIU/mL]/22.5).16,17
Body composition variablesAll patients underwent yearly evaluations of body composition with a HOLOGIC 2003 Explorer™ densitometer, from which we obtained measurements of total lean mass (LM), total fat mass (FM), percent lean mass and fat mass. We calculated the proportion of the fat mass and the lean mass relative to the sum of both (FM/[FM+LM]; LM/[FM+LM]) to assess changes in body composition over time.
Statistical analysisTo describe the baseline characteristics of the sample, we performed a descriptive analysis of the quantitative variables (mean, standard deviation, minimum, maximum, median and interquartile range) and expressed qualitative variables as frequency distributions.
We performed comparisons with parametric tests after verifying the normality of the distributions by means of the Kolmogorov–Smirnov and Shapiro–Wilk tests, and the homogeneity of variance by means of Levene's test.
To describe the changes that occurred during the years of followup, we compared means by repeated measures ANOVA. When we found significant differences in the ANOVA, we used Student's t test to compare different ranges in order to determine the time at which the changes occurred. We used Student's t test for independent samples to make comparisons between the sexes. We defined statistical significance as P<.05 in any of these tests.
The statistical analysis was performed with the software SPSS version 20.0 for Windows.
The study was approved by the Clinical Research Ethics Committee of Aragón (Comité Ético de Investigación Clínica en Aragón [CEICA]) of the Department of Health and Consumer Affairs of the autonomous community of Aragón. We also obtained the informed consent of patients and family members.
ResultsBaseline characteristicsThe sample included twenty-eight participants: twenty-one female (75%) and seven male (25%). At the beginning of the study, 78.5% of the sample was in stage 1 of the Tanner scale, and 21.5% in stage 2. The bone age to chronological age ratio was 0.70±0.522. The mean administered dose of rhGH was 0.029mg/kg/day.
- –
At birth: the mean gestational age was 38.29±3.46 weeks, and the mean auxologic measurements were 46.13±0.39cm for length (−2.42±0.20 SD) and 2575±85.63g for weight (−1.38±0.23 SD). The mean genetic potential length was −1.44±0.12 SD. We did not find significant differences in any of these variables based on sex. The documented aetiologies that may have contributed to intrauterine growth restriction were environmental factors in 35.7% (exposure to smoke and maternal employment), maternal factors in 10.7%, foetal factors in 10.7% and placental factors in 7.1%, with growth restriction considered idiopathic in 35.7%.
- –
At treatment initiation: Table 1 presents the anthropometric, laboratory and body composition data. The mean age at initiation was 8.79±3.06 years. The mean height was −2.76±0.45 SD, with a growth velocity of −1.43±1.79 SD. Blood pressure and waist circumference values were within the normal range, as were the baseline blood test results. We only found increased insulin levels and HOMA indices in female participants, which did not reach the levels associated with insulin resistance (insulin in male patients, 5.07±2.42 vs female patients, 10.59±11.01 [P=.042]; HOMA index in male patients, 1.89±0.98 vs female patients, 4.13±4.32 [P=.037]). Table 2 shows that there were significant differences in these two variables when they were analysed taking pubertal maturation into account (prepubertal insulin, 6.51±4.98mIU/mL vs pubertal insulin, 19.13±16.41mIU/mL [P=.003]; prepubertal HOMA index, 2.54±2.09 vs pubertal HOMA index, 7.32±6.42 [P=.005]).
Table 1.Anthropometric, clinical and laboratory data of the sample at baseline.
Mean (±SD) Minimum Maximum Median Q1 Q3 Pa Anthropometric data Age (years) 8.79±3.06 4.62 14.07 9.73 7.04 12.42 ns Weight (SD) −1.50±0.50 −2.68 −0.83 −1.21 −1.52 −0.9 ns Height (SD) −2.76±0.45 −4.90 −2.23 −2.77 −2.98 −2.56 ns BMI (SD) −0.68±0.68 −1.89 −0.91 −0.68 −1.16 −0.20 ns GV (SD) −1.43±1.79 −5.72 2.26 −1.47 −2.35 −0.59 ns Clinical data SBP (mmHg) 96.71±11.91 69 124 95 86.88 103.12 .022* SBP (SD) 0.20±1.21 −1.88 3.16 0.59 −0.15 1.33 .010* DBP (mmHg) 57.64±9.63 40 80 58 52 64 ns DBP (SD) 0.08±0.86 −1.50 2.14 0.08 −0.43 0.59 ns Waist circumference (cm) 60±11.01 44 85 55 47.50 62.50 ns Waist circumference (SD) −0.22±1.46 −2.50 3.50 −0.51 −1.10 0.08 ns Laboratory data Glucose (mg/dL) 85.67±8.88 65 100 89 84 94 ns Glycosilated Hb (%) 5.01±0.49 4.00 6.50 5.10 4.80 5.40 ns Triglycerides (mg/dL) 60.11±21.90 31 115 63 46 80 ns Colesterol total/HDL 2.72±0.62 1.50 4.30 2.80 2.49 3.11 ns Insulin (mIU/mL) 9.33±11.26 2.00 40.07 7.79 2.66 12.92 .042* IGF1 (ng/mL) 232.82±166.66 59 601 206 72 340 ns IGF1BP3 (μg/mL) 4.77±1.31 2.79 7.13 5.09 3.85 6.33 ns IGF1/IGF1BP3 49.14±41.31 14.73 200.30 41.54 19.49 63.59 ns HOMA index 3.63±4.42 0.58 15.99 3.12 0.74 5.50 .037* DEXA Fat mass (g) 6214.27±4540.16 2581.00 18,832.60 5418.30 2508.70 8327.90 ns Fat mass (%) 25.94±6.09 16 40.80 24 19.5 28.5 ns Lean mass (g) 16,303±5686.05 8669.9 29,080.50 16,589.20 11,602.60 21,575.80 ns Lean mass (%) 70.75±6.09 55.90 80.70 72.70 68.40 77 ns FM/[FM+LM] 26.81±6.90 16.05 40.80 25.44 20.93 29.95 ns LM/[FM+LM] 73.19±6.90 59.20 83.95 74.56 70.05 79.07 ns Data available for 28 patients.
BMI, body mass index; DBP, diastolic blood pressure; DEXA, body composition; FM, total fat mass; GV, growth velocity; LM, total lean mass; ns, not significant; SD, standard deviation; SBP, systolic blood pressure.
Table 2.Laboratory results at baseline by stage of pubertal development.
Prepubertal (Tanner I) Pubertal (Tanner II) Pa n=22 n=6 Sex % male 27.27 16.66 % female 72.72 83.33 Laboratory test results at baseline Glucose (mg/dL) 85.32±8.31 85.17±6.40 ns Glycosilated Hb (%) 4.97±0.54 5.15±0.26 ns Triglycerides (mg/dL) 58.36±16.47 64.67±28.64 ns Total cholesterol/HDL 2.74±0.62 2.58±0.47 ns Insulin (mIU/mL) 6.51±4.98 19.13±16.41 .003** IGF1 (ng/mL) 213.96±136.66 305.17±177.95 ns IGF1BP3 (μg/mL) 4.62±1.22 5.22±1.16 ns IGF1/IGF1BP3 48.04±37.97 54.86±24.99 ns HOMA index 2.54±2.09 7.32±6.42 .005** Data available for 28 patients.
Table 3 shows the evolution of the different variables.
Changes in anthropometric, clinical and laboratory data during the four years of treatment with growth hormone.
| Baseline (mean±SD) | 1st year (mean±SD) | 2nd year (mean±SD) | 3rd year (mean±SD) | 4th year (mean±SD) | Pa | |
|---|---|---|---|---|---|---|
| n=28 | n=27 | n=26 | n=24 | n=20 | ||
| Anthropometric data | ||||||
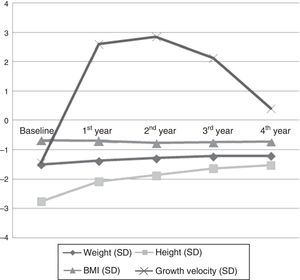
| Weight (SD) | −1.50±0.50 | −1.38±0.40 | −1.28±0.42 | −1.22±0.37 | −1.21±0.49 | .016* |
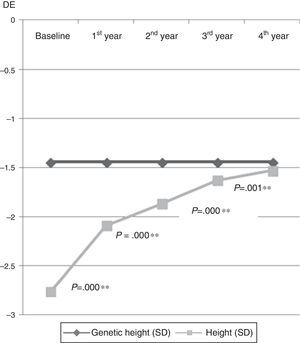
| Height (SD) | −2.76±0.45 | −2.09±1.03 | −1.87±0.60 | −1.63±0.60 | −1.53±0.62 | .000** |
| BMI (SD) | −0.68±0.68 | −0.69±0.46 | −0.77±0.52 | −0.74±0.43 | −0.72±0.52 | .585 |
| GV (SD) | −1.43±1.79 | 2.61±1.48 | 2.85±2.93 | 2.13±3.37 | 0.41±1.52 | .009** |
| Clinical data | ||||||
| SBP (mmHg) | 96.71±11.91 | 99.07±11.40 | 104.19±13.47 | 105.67±13.18 | 108.85±11.02 | .000** |
| SBP (SD) | 0.20±1.21 | 0.13±0.88 | 0.38±1.04 | 0.22±1.13 | 0.61±1.11 | .412 |
| DBP (mmHg) | 57.64±9.63 | 56.92±8.63 | 59.69±7.85 | 58.25±7.74 | 58.40±7.25 | .981 |
| DBP (SD) | 0.08±0.86 | −0.14±0.75 | −0.16±0.66 | −0.26±0.64 | −0.17±0.78 | .076 |
| Waist circumference (cm) | 60±11.01 | 62.88±7.76 | 64.38±7.40 | 67.00±9.72 | 62.61±7.74 | .280 |
| Waist circumference (SD) | −0.22±1.46 | −0.61±0.84 | −0.57±0.93 | −0.71±1.33 | −0.63±1.04 | .860 |
| Laboratory data | ||||||
| Glucose (mg/dL) | 85.67±8.88 | 87.46±8.35 | 86.92±8.76 | 84.14±9.86 | 87.43±6.28 | .542 |
| Glycosilated Hb (%) | 5.01±0.49 | 5.21±0.38 | 5.24±0.21 | 5.29±0.25 | 5.30±0.30 | .002** |
| Triglycerides (mg/dL) | 60.11±21.90 | 70.88±23.33 | 71.96±27.86 | 76.50±31.45 | 68.21±38.81 | .095 |
| Colesterol total/HDL | 2.72±0.62 | 2.68±0.51 | 2.77±0.53 | 2.64±0.57 | 2.60±0.60 | .859 |
| Insulin (mIU/mL) | 9.33±11.26 | 9.74±6.39 | 10.82±6.64 | 11.71±7.08 | 16.55±6.22 | .044* |
| IGF1 (ng/mL) | 232.82±166.66 | 358.35±173.68 | 463.08± 196.05 | 518.23±184.59 | 574.65±156.28 | .000** |
| IGF1BP3 (μg/mL) | 4.77±1.31 | 5.52±1.21 | 6.05±1.38 | 6.31±1.15 | 6.64±0.90 | .000** |
| IGF1/IGF1BP3 | 49.14±41.31 | 63.95±26.12 | 75.82±28.21 | 81.88±27.95 | 88.84±28.70 | .000** |
| HOMA index | 3.63±4.42 | 3.65±2.61 | 4.23±2.82 | 4.47±2.88 | 6.43±2.55 | .042* |
| DEXA | ||||||
| Fat mass (g) | 6214.27±4540.16 | 6439.33±2975.38 | 7664.18±2582.16 | 8121.49±2905.37 | 8346.57±2293.24 | .234 |
| Fat mass (%) | 25.94±6.09 | 23.91±4.39 | 23.82±4.43 | 22.45±5.42 | 21.04±4.81 | .015* |
| Lean mass (g) | 16,303±5686.05 | 19,319.10±5335.78 | 24,180.49±6776.29 | 27,538.93±7133.92 | 31,153.61±7214.24 | .000** |
| Lean mass (%) | 70.75±6.09 | 72.79±4.38 | 72.87±4.43 | 73.81±5.38 | 74.92±4.62 | .034* |
| FM/[FM+LM] | 73.19±6.90 | 75.62±6.02 | 75.94±4.39 | 77.09±5.52 | 78.74±4.33 | .037* |
| LM/[FM+LM] | 26.81±6.90 | 24.37±6.02 | 24.06±4.39 | 22.88±5.39 | 21.26±4.33 | .021* |
BMI, body mass index; DBP, diastolic blood pressure; DEXA, body composition; FM, total fat mass; GV, growth velocity; LM, total lean mass; SD, standard deviation; SBP, systolic blood pressure.
Anthropometric variables (Fig. 1): there was a gradual increase in height through time (from −2.76±0.45 SD to −1.53±0.62 SD; P=.000), with significant differences between all studied years. Fig. 2 shows how actual height gradually approached the genetic potential, and how the largest height gain occurred in the first year of treatment.
Weight improved as years passed (from −1.50±0.50 SD to −1.21±0.49 SD; P=.016). Growth velocity increased progressively (from −1.43±1.79 SD to 0.41±1.52 SD; P=.009). We found no changes in body mass index.
There were no differences between sexes in any of the variables.
Clinical data: we found no changes in systolic blood pressure with time, although we did find significant changes in diastolic blood pressure, which decreased in the second year of treatment (−0.16±0.13 SD vs −0.26±0.13 SD; P=.037). There were no changes in waist circumference. We found no differences in the variables based on sex.
Laboratory data: the percent glycosilated haemoglobin increased significantly, although it remained within the normal range, with a baseline of 5.01±0.49% and a value of 5.30±0.30% in the fourth year (P=.002). The largest increase occurred in the first year of treatment.
The plasma insulin levels increased significantly with the years, with levels of 9.33±11.26mIU/mL at baseline and of 16.55±6.22mIU/mL in the fourth year (P=.044), which were within normal ranges. We did not observe differences based on sex during the followup, as had been the case of the baseline levels.
The values of IGF1, IGF1BP3 and their ratio increased significantly, with the increase occurring in the first and second years.
The HOMA index increased significantly from 3.63±4.42 to 6.43±2.55 (P=.042), probably due to the increase in plasma insulin, as we did not observe any changes from baseline in glucose levels. We observed no changes in triglyceride levels during the followup. There were no differences between the sexes in any of the analysed variables.
Body composition data: lean mass increased significantly during the followup (from 16 303±5686.05g to 31 153.61±7214.24g; P=.000). There was a progressive increase in lean mass due to the growth of the patients, but there were no significant changes. Furthermore, we observed a significant increase of the percent lean mass attributable to changes that occurred in the first year of treatment (from 70.75%±6.09% to 74.92%±4.62%; P=.0034) as well as a decrease in the percent fat mass (from 25.94%±6.09% to 21.04±4.81%; P=.015).
The LM/[LM+FM] ratio increased during the followup from 73.19±6.90 to 78.74±4.33 (P=.037), a trend that was more pronounced in male patients. Since they are complementary, the FM/[LM+FM] decreased. When we analysed by time range, we found that the statistically significant differences were due to the changes that occurred in the first year of treatment.
DiscussionSmall for gestational age children are at risk of developing multiple diseases in adulthood.18 Most of these children experience catch-up growth and reach their genetic potential by age 4 years. However, due to an energy-conserving metabolism, these patients experience changes in body composition with an increase in BMI19 attributable to an increase in central fat mass, with accumulation of fat in the abdomen.20,21 This determines a series of changes, such as insulin resistance, that predisposes to other diseases during adulthood,22 like cardiovascular disease, diabetes or metabolic syndrome.23 There is evidence that patients with rapid spontaneous growth are at greater risk than patients treated with rhGH.24–26 Soto et al.26 described the presence of insulin resistance in children born SGA that experienced catch-up growth and its absence in children that did not experience this growth, suggesting that rapid catch-up growth can have a considerable impact on the development of metabolic disorders.
The patients included in our study were eligible for treatment with rhGH, as they had not experienced catch-up growth. Before initiating treatment, we observed that the laboratory results for metabolic parameters were within normal ranges. We ought to underscore that we found differences in insulin levels and the HOMA index when we analysed by stage of pubertal development, with pubertal patients having higher values. The differences observed between sexes could be explained by the higher proportion of female patients that were in the pubertal stage.
In this study, the analysis of anthropometric variables during the followup revealed a significant increase in growth velocity, more marked in the first year, leading to increases in final height that approximated the stature genetic potential. We also observed the normalisation of body weight, which was below the lower bound of the normal range at baseline. These findings are consistent with the previous literature.1,10
While they were always within normal ranges, we also observed a progressive increase in insulin, glycosilated haemoglobin and HOMA index values, which indicated a certain tendency towards insulin resistance. This was already described by Lebl et al., who also found a normalisation of these variables after treatment was discontinued.27 It is important that we continue the followup of these patients to evaluate the relevance of these increases in carbohydrate metabolism parameters and see whether they are sustained over time or revert once treatment is discontinued, as has been described in the past.
There is evidence of a GH dose-dependent response in the increase of IGF1 and IGFBP3 levels.28 Both increased throughout the follow-up period, with a greater increase in IGF1 that led to an increase in the IGF1/IGF1BP3 ratio. The levels remained within normal ranges during the entire followup. There is no clear international consensus on the upper bounds of the normal range, raising concerns on the potential impact of elevated levels in the long term.29
We did not find significant changes in systolic blood pressure, BMI, waist circumference or lipid metabolism. We did observe a decrease in diastolic blood pressure. Previous studies have already reported that treatment with rhGH is effective in normalising blood pressure values, both systolic and diastolic, in patients with a history of hypertension,30,31 which may contribute to reducing the risk of cardiovascular disease associated with being born SGA. In our study, patients had normal blood pressure at baseline, which may be the reason why we did not observe any significant changes. Something similar occurred with the lipid profile, which was found to improve as early as the first year of treatment in other studies.30 In our study, the baseline values were already normal.
The body composition of SGA children is different to that of children born with size appropriate for gestational age: they have less body fat, less lean mass and a reduced bone mineral density.32 They have an increased fat mass to lean mass ratio, which is a risk factor for developing metabolic syndrome. The main strength of our study is the changes we found by analysing the body composition of the patients. The variation of the ratios under study demonstrates a significant increase in the proportion of lean mass relative to fat mass through time, which was more pronounced in male patients and in the first year of treatment. The changes we observed may decrease the metabolic disease risk associated with SGA, and possibly lead to a reduction in the incidence of cardiovascular problems in this group of patients during adulthood. This reinforces the importance of rhGH treatment in SGA children beyond the main goal of achieving higher final heights. For all of the above, it is essential that we continue the followup of these children in order to assess how these variables change over time after completion of treatment with rhGH.
ConclusionTreatment with rhGH in a group of SGA children that had not experienced catch-up growth was effective, as we observed clear height gains and weight normalisation with no associated changes in BMI. We found significant changes in body composition, with an increase in lean mass associated with a decrease in body fat. Furthermore, there was evidence of a certain impact on carbohydrate metabolism, with some values indicative of insulin resistance. We found no changes in the lipid profile. These findings warrant the continued followup of these patients until they reach their final heights in order to determine whether the changes are sustained through time and assess their long-term consequences.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Aurensanz Clemente E, Samper Villagrasa P, Ayerza Casas A, Ruiz Frontera P, Bueno Lozano O, Moreno Aznar LA, et al. Modificaciones en variables antropométricas, analíticas de riesgo metabólico y composición corporal en pequeños para la edad gestacional en tratamiento con hormona de crecimiento. An Pediatr (Barc). 2017;86:240–248.
Previous presentations: The contents of this article were presented in poster format at the Congreso Extraordinario de la AEP and the II Congreso Extraordinario Latinoamericano de Pediatría; June 5–7, 2014; Madrid, Spain. The poster was awarded the en Madrid del 5 al 7 de junio del 2014, which was awarded a honourable mention for the best poster in the congress.