This is a consensus document of the Sociedad Española de Infectología Pediátrica, Sociedad Española de Reumatología Pediátrica and Sociedad Española de Ortopedia Pediátrica on the aetiology and diagnosis of uncomplicated acute osteomyelitis and septic arthritis.
A review is presented of the aetiopathogenesis and pathophysiology of acute osteoarticular infection defined as a process with less than 14 days of symptomatology, uncomplicated, and community-acquired. The diagnostic approach to these conditions is summarised based on the best available scientific knowledge. Based on this evidence, a number of recommendations for clinical practice are provided.
Se presenta el Documento de Consenso sobre etiopatogenia y diagnóstico de la osteomielitis aguda y la artritis séptica no complicadas elaborado por la Sociedad Española de Infectología Pediátrica, la Sociedad Española de Reumatología Pediátrica y la Sociedad Española de Ortopedia Pediátrica.
En este documento se revisan la etiopatogenia y la fisiopatología de la infección osteoarticular aguda en niños, considerada como aquella no complicada, de origen comunitario, que presenta una evolución inferior a 14 días, así como la aproximación clínico-diagnóstica a estas entidades, basándonos en las mejores evidencias científicas disponibles. En función de dichas evidencias, se aportan una serie de recomendaciones para la práctica clínica.
Osteoarticular infection (OAI) is particularly important because the musculoskeletal system of the child is constantly growing. Thus, an infection that affects the growth cartilage or epiphyseal plate may cause abnormalities in bone development with the corresponding sequelae. The three participating societies, the Sociedad Española de Infectología Pediátrica (Spanish Society of Paediatric Infectology [SEIP]), the Sociedad Española de Reumatología Pediátrica (Spanish Society of Paediatric Rheumatology [SERPE]) and the Sociedad Española de Ortopedia Infantil (Spanish Society of Childhood Orthopaedics [SEOP]) consider that it is very important to have a consensus document gathering all the published scientific evidence on uncomplicated OAIs in the paediatric age group. This document covers both acute osteomyelitis (AOM) and septic arthritis (SA), and is essentially a review of the evidence on haematogenous community-acquired infections with an acute course (<14 days). We have also developed a set of recommendations that we present with their corresponding levels of evidence and grades of recommendation (Table 1).1
Levels of evidence and strengths of recommendation used in this consensus paper.
| Category | Definition |
|---|---|
| Strength of recommendation | |
| A | Good evidence |
| B | Moderate evidence |
| C | Poor evidence |
| Quality of evidence | |
| I | Properly randomised controlled trial |
| II | Well-designed clinical trial without randomisationCohort studyCase-control studyOther: multiple time series or dramatic results from uncontrolled experiments |
| III | Expert opinions based on clinical experienceDescriptive studiesRecommendations of expert committees |
This consensus is not intended to replace the clinical judgement or to serve as a protocol to be applied to all children with this type of infection, and its contents are probably not the only suitable approach to OAIs in children.
A future document will address the treatment of OAIs.
EpidemiologyOAIs are most frequent in the paediatric age group, with a prevalence of 22 cases per 100,000 children in developed countries.2 Fifty percent of cases occur in children younger than 5 years, and at least 25% in children younger than 2 years.3 The annual incidence of SA is estimated at 4 cases per 100,000 children,4 and that of AOM at 2–13 cases per 100,000 children,5,6 with the latter being twice as frequent. When it comes to neonatal osteomyelitis, the reported incidence in intensive care units is of 1–3 cases per 1000 admissions.7 The male to female ratio is 1.2/3.7.7
The literature describes a 2.8 fold increase in the number of AOM cases in the past 20 years while the number of SA cases has remained stable,5,6 a trend that may be due to advances in diagnostic techniques. There are several predisposing factors that promote the development of OAIs, which are detailed in Table 2, although most OAIs occur in children with no underlying disease.
Predisposing factors that promote the development of an osteoarticular infection.
| Primary immunodeficiencies such as chronic granulomatous disease, Wiskott-Aldrich syndrome or Chediak-Higashi syndrome |
| Infection by human immunodeficiency virus |
| Haemoglobinopathies, especially sickle-cell anaemia |
| Sepsis |
| Trauma with bacteraemia (responsible for 30% of AOM cases) |
| Varicella |
| Surgery (joint, intestinal or urinary tract) |
| Puncture wounds, skin infections or presence of foreign bodies |
| Haemodyalisis |
| Diabetes |
| During the neonatal period |
| Preterm birth |
| Skin infections |
| Umbilical venous or central venous catheterisation |
| Previous infections, especially those associated with bacteraemia (or fungaemia) |
| Occasionally, AOM has developed following heel puncture with a lancet |
Usually, AOM is unifocal and affects the metaphyses of long bones, especially femur (30%), tibia (22%) and humerus (12%),7,8 while involvement of the calcaneus or the pelvis is less frequent. The reported prevalence of AOM of the hip ranges between 1% to 11%, and it usually affects older children.8,9 Multifocal infections are more frequent in newborns (NBs), with a prevalence of up to 40%, in children with immunodeficiencies, and in children infected by methicillin-resistant Staphylococcus aureus (MRSA).9,10
When it comes to SA, over 90% of the cases are monoarticular3 and they affect lower limb joints most frequently (70% of cases), especially the knee (35–40%), hip (25–30%), ankle (13–15%), elbow (10%) and shoulder (5%).3,4,11
OAIs infections of the spine may cause discitis, and most frequently affect the lumbar region7,12,13 and children younger than 5 years as intervertebral discs are vascularised from the adjacent vertebra (a vascularisation that disappears later on).7,8,12 Cases of discitis described in adolescents may be due to avascular necrosis as opposed to true infection. Vertebral involvement occurs in 1–2% of cases, especially in children more than 8 years of age.
AOM and SA (osteoarthritis) coexist in up to 30% of children (especially NBs [70% of cases] and children less than 18 months of age), mainly at the shoulder or hip due to the presence of intra-articular metaphyses.7,8,12
AetiologyMost frequent bacterial agentsThe most frequent microorganism in every age group is Staphylococcus aureus (S. aureus). In NBs and up to 3 months of age, Streptococcus agalactiae and enterobacteria (especially Escherichia coli) are also important. Between 3 months and 2–5 years of age, the most frequent causative agents are S. aureus and Kingella kingae, while from this age onward most infections are caused by S. aureus and, to a lesser degree, by Streptococcus pyogenes14 (Table 3). When arthritis is found in sexually active adolescents, Neisseria gonorrhoeae should also be considered.11Table 3 presents the bacteria associated with different risk factors.
Most frequent aetiology of osteoarticular infection by age and associated risk factors.
| Age | Bacteria |
|---|---|
| <3 monthsa | Staphylococcus aureusStreptococcus agalactiaeEnterobacteria (especially Escherichia coli) |
| 3 months–5 yearsb | S. aureusKingella kingaeS. pyogenes |
| >5 yearsc | S. aureusS. pyogenes |
| Risk situation | Bacteria |
|---|---|
| Puncture wound in foot through sports shoes | Pseudomonas aeruginosa |
| Varicella and wounds | S. pyogenes |
| Sickle-cell anaemia | Salmonella enteritidis |
| Complement deficiency | Neisseria meningitidisd |
| Newborns with complex diseases, immunodeficiencies, patients with prostheses or osteosynthesis devices | Coagulase-negative Staphylococci; S. epidermidis, S. hominis, S. saprophyticus, S. haemolyticus, S. lugdunensis. Candida spp, and other cocci and gram-positive and gram-negative bacilli |
| Agammaglobulinaemia | Mycoplasma pneumoniae |
| Chronic granulomatous disease | S. aureus, Serratia marcescens and Aspergillus fumigatus, among others |
| Patients from countries highly endemic for tuberculosis, immunodeficiencies that affect the interferon gamma and interleukin-12 pathway and treatment with biological immunomodulators that interfere with interferon synthesis | Mycobacterium tuberculosis |
Other microorganisms occasionally associated with osteoarticular infection in newborns are: N. gonorrhoeae, coagulase-negative Staphylococcus or Candida.
Most cases of SA are caused by the haematogenous spread of microorganisms to the synovium. Bacterial endotoxins in the joint space stimulate the release of cytokines, leading to leucocyte migration and the destruction of the cartilage matrix within the joint.11 When it comes to the hip and shoulder, this effect is compounded by the damage caused by the vascular collapse that results from the increased intra-articular pressure exerted by the accumulation of pus.
Infection may also spread from adjacent foci, especially in cases of osteomyelitis in infants.13 Lastly, the joints may become infected from puncture wounds, arthrotomies or the injection of drugs in the joint space, with the last two causes being rare.15
Acute osteomyelitisThe most frequent source of infection is the haematogenous route. AOM originating from open fractures, puncture wounds, animal bites or contiguous infections such as sinusitis, tooth infections or mastoiditis are less frequent.
In the context of bacteraemia, infection spreads to long-bone metaphyses due to their abundant blood supply: the slow blood flow in their capillary loops and the presence of endothelial gaps allow the passage of microorganisms. The bacteria proliferate, forming colonies that obstruct the capillary lumina, hindering phagocytosis and antibiotic penetration. The foci of metaphyseal infection become the source of bone marrow and cortical bone infection. The presence of transphyseal vessels in NBs and infants provides a vascular connection between the metaphysis and the epiphysis, promoting the development of osteoarthritis in these age groups. Since metaphyseal capillaries atrophy at 18 months, joint involvement is rare in later ages except for joints with intracapsular metaphyses like the hip or the shoulder.13
S. aureus, the most frequent pathogen in OAIs, has numerous surface proteins responsible for its adhesion to host tissues. Once adhesion has been achieved, the ability to form biofilms or small colonies contributes to the persistence of the infection. In addition, S. aureus can produce proteins that inhibit chemotaxis, leukocidins (Panton-Valentine leukocidin [PVL]) that destroy leukocytes and modulins that can induce lysis in osteoblasts.16,17
Clinical manifestationsGeneral symptomsThe duration of symptoms of an acute OAI is less than 14 days; when the duration is longer is considered subacute or chronic. This duration is arbitrary, albeit widely accepted, and additional radiological, clinical or pathological anatomy criteria may be important to better define the infection.
The initial symptoms of OAI may be nonspecific, such as irritability, general malaise, decreased appetite or decreased activity. Patients, especially NBs and young infants, may not always present with fever,6,9 which is found in 62–72% of cases18 and it is more frequent in SA.11,19
Local symptomsThe main clinical features are focal pain and decreased range of motion or functional impairment, which are found in 56–95% and 50–92% of cases, respectively.6,20 Frequently, the patient develops an antialgic posture. When the infection is localised in the lower limbs or the axial skeleton (spondylodiscitis, sacroiliitis), the patient limps or refuses to walk. Other local symptoms will depend on the site of infection:
- –
In addition to pain, AOM may present with swelling, warmth, and even erythema if the infection has spread to the subperiosteal space and subcutaneous tissue.6
- –
When infection in SA is superficial, for instance at the knee, it manifests with pain, swelling and warmth, and seldom with erythema. However, when it is deep, as occurs in hip infections, there is no swelling, warmth or redness,9 and clinical suspicion arises from the report of pain in the ipsilateral groin, thigh or knee combined with a painful reduction of mobility in the joint, especially in internal rotation: the child usually displays an antalgic position of the hip, with sustained flexion, external rotation and abduction.
- –
Patients with spondylodiscitis and sacroiliitis avoid walking and standing, and assume a characteristic tripod position. A high degree of suspicion is required to diagnose OAI at these locations.9
The most benign and frequent form manifests without systemic symptoms or fever, and the typical presentation consists of irritability, decreased appetite and pseudoparalysis of the affected limb with pain upon movement.8 There is a severe form that presents with bacteraemia and typical signs of sepsis that occurs more frequently in preterm NBs.8,21 There are also cases of AOM secondary to cephalohaematoma or invasive monitoring.12
Sickle-cell diseaseThese children are at high risk of developing AOM and SA due to microvascular occlusions.11,22 The clinical presentation of AOM is similar to that of a vaso-occlusive crisis and the differential diagnosis can be quite challenging. Infection episodes seem to be associated with higher fever and longer duration of pain at a single location.23
Physical examinationThe physical examination is key to establishing diagnostic suspicion and subsequently assessing the course of the disease. It consists of:
- –
Detailed general examination, starting from the contralateral side.
- –
Observation of spontaneous posture.
- –
Assessment of the musculoskeletal system, which may be performed by means of the pGALS (paediatric gait, arms, legs, spine), a validated and easy-to-implement tool. Table 4 presents a simplified version of the pGALS.24,25
Table 4.The pGALS (paediatric gait, arms, legs, spine) assessment for detecting musculoskeletal symptoms.
Questions Does the child have any pain or stiffness (numbness after being at rest) in the joints, muscles or back? Does the child have any difficulty getting him or herself dressed without any help? (if child used to be able to do it before) Does the child have any difficulty going up and down stairs? (if child used to be able to do it) Screening manoeuvres What is being assessed? Observe the patient standing and lying down Spontaneous posture. Exanthema. Leg length inequality or dysmetria. Joint swelling. Valgus/varus deformity. Muscle wasting. Flat feet Upper limbs “Hold your hands out straight in front of you” Anterior flexion of shouldersElbow, wrist and finger extension “Turn your hands over and make a fist” Elbow supinationFlexion of finger joints “Pinch your index finger and thumb together” Manual dexterity “Touch the tips of your fingers with the thumb of the same hand” Manual dexterity “Put your hands together palm to palm at elbow level” Extension of wrist and finger joints “Reach up with your arms and stretch as far as you can” Elbow and wrist extensionShoulder abduction “Put your hands behind your neck” Shoulder abduction and external rotationElbow flexion Pain upon squeezing the knuckles Metacarpophalangeal joints Lower limbs Perform patellar tap (perhaps bouncing) Knee stiffness “Extend and flex the knees” Knee extension and flexion Passive movement of hips (knee flexed to 90° and internal rotation of the hip in the prone position: it is the most sensitive manoeuvre for the assessment of the hip) Hip rotation, abduction and flexion Perform passive ankle movements Mobility of talocrural and subtalar joints Spine and temporomandibular joints “Open your mouth wide” Temporomandibular joints and deviation of jaw opening “Look at the ceiling” Cervical spine extension “Try to touch your shoulder with your ear” Cervical spine lateral flexion “Stand up with straight knees and try to touch the floor” Flexion of thoraco-lumbar spineScoliosis Gait Observe spontaneous gait“Walk on your toes”“Walk on your heels” Limping or refusal to walk Adapted from Goff et al.24 - –
Comparison of the affected side with the healthy contralateral side to identify differences in posture, size, temperature and colour.
- –
Assessment of the gait.
The Fabere manoeuvre can be useful in the assessment of the sacroiliac joint (http://www.aeped.es/sites/default/files/documentos/cojera.pdf).
We want to highlight that: (1) Pinpoint tenderness is typical of AOM; (2) manoeuvres for the assessment of sacroiliitis, such as the Fabere test, are not useful in young children because they are uncooperative, and (3) erythema supra-adjacent to a joint is a sign of soft-tissue involvement and usually does not correspond to a true case of arthritis.
Diagnosis of osteoarticular infectionsLaboratory and microbiological diagnosisClinical suspicion, history taking and physical examination are the first steps to achieve the diagnosis. Additional tests will help confirm the diagnosis.
- –
Complete blood count and acute phase reactants: there is frequently an increase in the erythrocyte sedimentation rate (ESR) and the levels of C-reactive protein (CRP) and other acute phase reactants, although these are nonspecific findings. These parameters are not usually very elevated except at advanced stages. The combined elevation of CRP and ESR is very useful for the initial assessment, as OAI is improbable if these values are not elevated soon after admission.26 Leukocytosis is rarely found.19
- –
Blood culture: a sample must always be collected to attempt the identification of the causative microorganism, although its diagnostic yield is less than 50%.27
- –
Analysis of joint fluid: arthrocentesis or the puncture of the joint space to collect synovial fluid should be performed whenever SA is suspected and before initiating antibiotic treatment. Thus, the cytological and biochemical analysis of synovial fluid, while nonspecific, can help narrow down the diagnosis (Table 5).15 Usually, a white blood cell count above 50,000cells/mm3 with polymorphonuclear cell predominance is suggestive of an infectious aetiology, as it is a reduced glucose level (<50% of the plasma level).
Identification of the causative agent is the gold standard for SA diagnosis, although it is only achieved in 20–80% of cases.6,28 Ideally, all the fluid or tissue collected in a sterile tube (swabs should not be used) should be sent as soon as possible to be processed for gram staining and culture. The direct inoculation of joint fluid in a blood culture bottle increases the yield, especially when K. kingae is suspected (possibly the most frequent agent in negative-culture OAI).7,29 Polymerase chain reaction (PCR) allows the identification of this and other bacteria that grow poorly in culture and in infections that have been previously treated with antibiotics.6,14,30–32To date, we have not found biological and clinical characteristics of SA that would allow the prediction of the involved pathogen,33,34 except for younger age, milder symptoms and lower levels of inflammatory markers in children infected by Kingella.19,32,34
Additional specimens for acid-fast staining, culture and PCR should be collected for the diagnosis of tuberculous arthritis; in this case a synovial biopsy may also be required.35
- –
Joint puncture: some authors believe that it is not needed before starting empirical antibiotic therapy in AOM, reserving it for patients with unfavourable outcomes, those with abscesses or with chronic osteomyelitis.6,19 However, some facilities perform it regularly and without complications, with or without ultrasound aid and it leads to the identification of the microorganism in 40–60% of the cases.7
- –
Tuberculin test (or immunological-based tests): it should be requested when osteoarticular tuberculosis is suspected, although this disease usually has a subacute or chronic course.
The diagnosis of other, less frequent infectious causes may require additional specific tests (PCR and serology).
Imaging tests- –
Plain radiography (X-ray). X-ray is still relevant when an OAI is suspected and it is useful in ruling out other pathologies such as fractures or tumours. In cases of AOM, X-rays are usually normal in the first 10–14 days, and then reach a sensitivity of 82% and a specificity of 92% at 2–3 weeks from the onset of symptoms. Some typical radiological features of AOM are osteolysis, osteopaenia and periosteal elevation or cortical thickening.36 In vertebral osteomyelitis or spondylodiscitis, the X-ray may show a reduction in the intervertebral space and a lateral X-ray may show vertebral erosions.7 In cases of SA, it may show an enlargement of the joint space and the soft tissues in the acute phase.
- –
Ultrasound. This is a very useful technique in SA due to its low cost, accessibility and high sensitivity. It detects joint effusion in 95% of cases, although the ultrasound findings are not pathognomonic for infection. It is especially relevant in the diagnosis of SA of the hip and shoulder due to the difficulty of a clinical diagnosis6,11 and it can be very helpful in guiding arthrocentesis. Doppler ultrasonography can show increased blood flow, although their absence does not rule out SA.
- –
In cases of AOM, it helps detect subperiosteal or soft-tissue abscesses, but a normal scan does not rule out this infection.6,7,37 It can also be used in guiding aspiration. In the early stages, a positive findings in Doppler ultrasound examination of the bone suggest the presence of AOM.
- –
Computed tomography (TC). Although it does not detect specific changes in the early stages, it can show soft-tissue oedemas and deep extraosseous abscesses, facilitating puncture and drainage. It can be useful in the diagnosis of infections of the pelvis or with subacute and chronic courses, as it can detect bone sequestrations.7 Nevertheless, at present it is not used often due to the high amount of radiation involved.
- –
Magnetic resonance imaging. It is probably the best imaging test for the diagnosis of AOM and it is quite useful in the investigation of lesions of the axial skeleton and the pelvis.7,12 If the location of the lesion is known, it has a high sensitivity (97%) and specificity (92%).7 However, it may be difficult to differentiate other lesions that manifest with tissue oedema, such as tumours, fractures, bone infarction or bone changes secondary to concussion, although this diagnostic issue does not usually arise in clinical practise. Due to its cost, the need of sedation in young children and its lower availability, its use is often restricted to cases with a protracted or complicated course. The main indications for MRI are: confirmation of osteomyelitis, suspicion of complications (abscess/sequestration), vertebral or pelvic osteomyelitis, inconclusive ultrasound or scintigraphy results in OAI and SA cases refractory to antibiotic therapy.
- –
Bone scintigraphy. Although it has a low specificity, it is a very sensitive technique for locating the site of osteomyelitis, sacroiliitis or spondylodiscitis and for ruling out multifocality. Lower sensitivities have been found in young infants and neonates, and in infections by very virulent bacteria such as community-acquired (CA) MRSA.7,38 Technetium-99 is the preferred isotope due to the ease and quickness of the technique but it may result in false positives in the presence of other conditions with osteoclastic hyperactivity, such as fractures, tumours, bone infarction or post-operative lesions.37,39 Gallium-67 or labelled leucocyte scanning are believed to be more specific in active infections, but these techniques are more complex and obtaining the images may take up to 48h.39
- –
Other techniques. Single-photon emission CT or bone marrow scintigraphy with sulphur colloid may have some diagnostic indications for this pathology in the future.
While in most instance the AOM diagnosis should be confirmed with an imaging test other than a simple radiograph, in mild clinical illnesses that respond well to treatment further imaging may not be necessary.
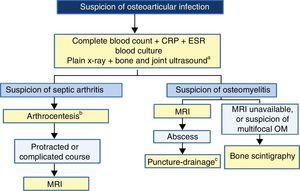
Fig. 1 presents an algorithm for the diagnosis of OAI.
Algorithm for the diagnosis of osteoarticular infection. a Ultrasound is very helpful in guiding the initial diagnosis of septic arthritis but not as helpful in the diagnosis of osteomyelitis (see text). b In septic arthritis of the hip or shoulder, joint decompression should be achieved as soon as possible (see text). c Puncture and drainage can be guided by ultrasound or computed tomography. ESR: erythrocyte sedimentation rate, MRI: magnetic resonance imaging, PCR: polymerase chain reaction.
Table 6 shows the most relevant differential diagnoses for monoarticular SA as well as their significant specific characteristics.25 Skin and soft-tissue infection or knee bursitis must also be considered. On rare occasions, SA is polyarticular and must be differentiated from rheumatic diseases, such as systemic lupus erythematosus, rheumatic fever or juvenile idiopathic arthritis, and from reactive arthritis secondary to certain infections11.
Differential diagnosis of monoarthritis.
| Age (years) | Sex | Synovial fluid | Key data | |||
|---|---|---|---|---|---|---|
| Appearance | Cell count (cells/mm3) | Culture or PCR | ||||
| Septic arthritis | <5 | M | Cloudy, purulent | >50,000 | (+) | Immobility due to pain, general malaise, acute onset |
| Viral arthritis | Yellow, clear | <10,000 | (−) | Usually polyarticularExanthema, leukopaenia. Serology (+) | ||
| Postinfectious arthritisa | Yellow, cloudy | >10,000 | (−) | Usually polyarticularHistory of pharyngitis or diarrhoea | ||
| JIA (oligoarticular) | <6 | F | Yellow, cloudy | >10,000 | (−) | Persistent arthritis. ANA (+) |
| JIA. ERA | >6 | M | Yellow, cloudy | >10,000 | (−) | Persistent arthritis and/or enthesitisHLA B27 (+) |
| Clotting disorder | Bloody | (−) | Haematomas in unusual locations | |||
| Tuberculous arthritis | Yellow, cloudy | 10–20,000 | (+) | Mantoux (+)Chest X-ray +/−. Epidemiological context | ||
| Villonodular synovitis | Bloody | (−) | Benign synovial proliferationbDiagnosis by biopsy | |||
| Synovial haemangioma | Bloody | (−) | Benign proliferation of synovial vessels. Diagnosis by MRI | |||
| Traumatic arthritis | >10 | Yellow or bloody | <2000 | (−) | Trauma/increased activity | |
| Transient synovitis of the hip | 3–9 | M | Arthrocentesis is not indicated | Suspicion based on clinical manifestationsPossibly, history of self-limited viral infectionSymptoms resolve in 5–7 daysRare in ages <3 years | ||
| Perthes disease | 3–9 | M | Arthrocentesis is not indicated | Can be suspected from clinical manifestationsHistory of limping episodesCompatible imaging studies | ||
ANA, antinuclear antibodies; ERA, enthesitis-related arthritis; F, female; JIA, juvenile idiopathic arthritis; M, male; MRI: magnetic resonance imaging; PCR, polymerase chain reaction.
The differential diagnosis takes into consideration: (1) cellulitis and soft-tissue infections, especially myositis (sometimes associated to AOM); (2) fractures and other traumatic injuries; (3) osteochondrosis, usually manifesting with local inflammatory signs with no increase in acute phase reactants and normal radiological appearance; (4) benign tumours (osteoid osteoma, osteoblastoma); (5) malignant tumours, such as Ewing sarcoma (soft tissue involvement, fever and general malaise), neuroblastoma (bone metastasis) and osteosarcoma; 6) aseptic inflammatory osteitis, such as chronic recurrent multifocal osteomyelitis (CRMO); (7) Langerhans cell histiocytosis, which may be clinically and radiologically indistinguishable from osteomyelitis; (8) lymphoproliferative disorders that may be mistaken for AOM due to the presence of pain and inflammation,8,12 and (9) osteonecrosis due to vaso-occlusive crises in sickle-cell disease.8,22 In the rare cases that there are multiple sites of infection (S. aureus, Bartonella, Coxiella), the differential diagnosis must include CRMO.
A bone biopsy and histological examination should be performed if the diagnosis is uncertain, and the specimen must be processed for culture and PCR determination of rarer microorganisms, such as fungi and mycobacteria. Table 7 presents the most relevant recommendations of this consensus along with their grades of evidence.
Summary of recommendations and evidence.
| – AOM and SA are more frequent in children less than 5 years of age (AI) |
| – 70% of cases involve the lower limbs, usually at a single location (AI) |
| – The most frequently isolated microorganism in all ages is S. aureus (AI). Other important pathogens in NBs and up to 3 months of age are S. agalactiae and enterobacteria (especially E. coli) (AI) |
| – K. kingae is especially frequent in children from 3 months to 5 years of age, being the second most common agent isolated at this age (AI) |
| – SA caused by Salmonella may occur, albeit rarely, in healthy children but it is more typical of immunosuppressed children, and especially in children with haemoglobinopathies such as sickle-cell disease. The duration of therapy should be, at least, 4–6 weeks (BIII) |
| – Low-grade fever may be eliminated. The most significant symptom is pain, which is the cause of the immobility of the affected site (AII) |
| – The diagnosis of OAIs is fundamentally based on clinical features, especially on physical examination. The diagnosis is supported by the levels of inflammatory markers, imaging studies and synovial fluid testing in SA (AII) |
| – Cytological and biochemical testing of joint fluid is important in the diagnosis of SA, although it is also nonspecific (BIII) |
| – The definitive diagnosis is established when the culture (joint fluid, blood, bone sample) or PCR results are positive (AI). If microbiological tests are negative, the diagnosis remains presumptive |
| – Arthrocentesis should always be performed in SA for the purposes of diagnosis and decompression of the joint space (AI). Cases of advanced SA or with highly organised tissue may require surgery for drainage and sample collection (BII) |
| – Although collection of a bone sample for microbiological diagnosis before initiation of antibiotic is a common practice in some facilities since it may guide the approach to AOM by increasing the diagnostic yield, this does not seem an indispensable practice in cases of uncomplicated haematogenous AOM (AII); however, it should be considered in cases that do not respond to antibiotic treatment within 48–72h (BII) and in nonhaematogenous AOM |
| – An initial plain radiograph of the suspected area involved should be performed given its availability, low cost and potential for detecting other underlying pathologies or chronic changes in followup examinations (AII) |
| – Ultrasonography is the most useful technique in the initial assessment of SA due to its high sensitivity for detecting increases in joint fluid, although the characteristics of joint effusion are nonspecific (AII) |
| – If AOM is suspected, radiological tests are normal and the lesion cannot be located based on the signs and symptoms, a bone scintigraphy should be performed (AII), especially when axial skeleton or small bone involvement are suspected |
| – MRI is the imaging study with the highest specificity for OAIs, especially for AOM (AI), and it can be very useful to rule out or confirm OAI when there are doubts in the diagnosis. It is usually not necessary in SA. In AOM, although it is not needed in every case, it can be very useful, especially in cases with axial skeleton involvement, protracted course or suspected complications (AII) |
The authors have no conflicts of interest to declare in relation to what is expressed in this document.
We want to thank the Grupo de Infecciones Osteoarticulares of the SERPE.
Please cite this article as: Saavedra-Lozano J, Calvo C, Huguet Carol R, Rodrigo C, Núñez E, Pérez C, et al. Documento de Consenso SEIP-SERPE-SEOP sobre etiopatogenia y diagnóstico de la osteomielitis aguda y artritis séptica no complicadas. An Pediatr (Barc). 2015;83:216.e1–216.e10.