The incidence of community-acquired pneumonia complications has increased during the last decade. According to the records from several countries, empyema and necrotising pneumonia became more frequent during the last few years. The optimal therapeutic approach for such conditions is still controversial. Both pharmacological management (antimicrobials and fibrinolysis), and surgical management (pleural drainage and video-assisted thoracoscopic surgery), are the subject of continuous assessment. In this paper, the Spanish Society of Paediatric Infectious Diseases and the Spanish Society of Paediatric Chest Diseases have reviewed the available evidence. Consensus treatment guidelines are proposed for complications of community-acquired pneumonia in children, focusing on parapneumonic pleural effusion. Recommendations are also provided for the increasing population of patients with underlying diseases and immunosuppression.
Desde hace más de una década, los casos complicados de neumonía adquirida en la comunidad, fundamentalmente con empiema pleural o formas necrosantes, comenzaron a ser más frecuentes en niños, según la amplia documentación procedente de numerosos países. El abordaje terapéutico óptimo de estos casos, tanto desde el punto de vista médico (antibióticos, fibrinolíticos) como técnico-quirúrgico, (drenaje pleural, videotoracoscopia) continúa siendo controvertido. En este documento, la Sociedad Española de Infectología Pediátrica y la Sociedad Española de Neumología Pediátrica revisan la evidencia científica y proponen unas pautas consensuadas de tratamiento de estos casos, fundamentalmente para el abordaje del derrame pleural paraneumónico en niños, así como la actuación en situaciones especiales, sobre todo en la cada vez más frecuente población pediátrica con enfermedades de base o inmumodepresión.
In the late 1990s there was a progressive increase in the number of complicated cases of community-acquired pneumonia (CAP), and particularly of cases complicated by pleural effusion.1,2 The trend continued over the following decade, with striking incidences of pleural empyema and necrotising forms of disease, especially of pneumococcal aetiology, in children older than 2 years.3–5 This epidemiological change was probably due to multiple factors, including the serotype shift in the nasopharyngeal carriage of pneumococcal strains due to the pressure of the 7-valent pneumococcal conjugate vaccine, although the trend was already apparent before the vaccine was introduced.1,2 This new situation mostly involved the emergence of several pneumococcal serogroups, such as 1, 3, 5 and 19A.3 However, it seems that the increasing trend has been partially curbed since the introduction in 2010 of new pneumococcal vaccines that cover the emerging serotypes, especially the 13-valent vaccine.6,7
Meanwhile, disease caused by Staphylococcus aureus (S. aureus) has been increasing at a slow pace, including disease by methicillin-resistant strains (MRSA) and strains that produce specific virulence factors, such as Panton-Valentine leukocidin (PVL), that can increase clinical severity, while the prevalence of other causative agents, such as Streptococcus pyogenes (S. pyogenes), continues to be low.8
This consensus document has been developed by the Sociedad Española de Infectología Pediátrica (Spanish Society of Paediatric Infectious Medicine [SEIP]) and the Sociedad Española de Neumología Pediátrica (Spanish Society of Paediatric Chest Diseases [SENP]), and it proposes guidelines for the treatment of complicated CAP and for the management of special circumstances, concluding the documents previously published in this journal on the diagnosis,8 treatment of uncomplicated cases, and prevention of CAP.9
The criteria for admitting children with CAP to the hospital may vary from one facility to another. This consensus offers recommendations approved by the authors of this document and supported by international guidelines, 10,11 which can be consulted in Table 1.
Criteria proposed for hospital and PICU admission of children with community-acquired pneumonia.
| Criteria for hospital admission |
| Clinical criteria |
| Septic appearance |
| Poor general health status |
| Moderate to severe tachypnoea |
| Retractions—use of accessory respiratory muscles (of any degree), suprasternal, intercostal or subcostal; grunting |
| Apnoeas |
| Sustained SaO2 below 92% with environmental air |
| Dehydration and/or significant electrolyte imbalance |
| Fatigue-somnolence |
| Cannot tolerate enteral nutrition |
| Oral antibiotherapy cannot be administered (vomiting, family cannot cooperate with treatment…) |
| Lack of response to appropriate empiric oral treatment 48h after initiation |
| Radiological criteria |
| Multifocal involvement in typical CAP |
| Lung abscess |
| Pneumatoceles |
| Significant pleural involvement |
| Severe interstitial lung pattern |
| Radiological images suggestive of an atypical microorganism |
| Risk factors to consider |
| Age <6–12 months |
| Underlying disease, including: |
| Malnutrition |
| Immunodeficiency |
| Cystic fibrosis |
| Bronchiectasis |
| Bronchopulmonary dysplasia associated with preterm birth |
| Cardiac disease |
| Renal disease |
| Diabetes |
| Inadequate hygiene-social environment. Inadequate supervision in the household |
| Criteria for admission to the paediatric intensive care unit |
| Shock |
| Severe breathing difficulty or respiratory muscle fatigue, in spite of supplementary oxygen |
| Frequent apnoeas |
| Hypoxaemia (SaO2≤90%) despite oxygen therapy with con FiO2≥0.5 |
| Progressive hypercapnia (pCO2≥65–70, capillary or venous) |
| Rapidly progressing radiological findings |
| Pneumothorax |
| Severe metabolic abnormalities |
| Changes in level of consciousness |
FiO2, fraction of inspired oxygen; pCO2, partial pressure of carbon dioxide; PICU, paediatric intensive care unit; SaO2, oxygen saturation.
While there is broader consensus on first-line antibiotic treatment, its choice should be determined on a case-by-case basis in a certain group of at-risk patients. There is greater controversy surrounding the best approach to the management of complicated cases with pleural effusion, both from a medical and a technical–surgical standpoint, which we will therefore analyse here.
Supportive careIn addition to antibiotic treatment, children admitted with CAP require supportive care, a subject that was partly addressed in the previous consensus document9 and that we proceed to complete in this one.
MonitoringPulse oximetry, which can be continuous. If the condition is serious, pCO2 should be measured, as hypercapnia is a sign of impending respiratory failure.12
Respiratory support- (A)
Oxygen therapy: with nasal cannulae, masks or face tents if the basal oxygen saturation (SaO2) is equal or lower than 92%, to achieve a FiO2 of up to 40% with the prongs or up to 50% with Venturi masks. If this were not sufficient, high-flow cannulae or non-rebreather masks with 100% oxygen should be used, assessing the need for intensive care unit admission in the following hours (Table 1) except in cases with limitation of therapeutic effort.
- (B)
Respiratory support: in exceptional cases, patients with CAP require mechanical ventilation. There is growing evidence of the advantages of non-invasive ventilation.13
Baseline requirements must be met by either the oral or, when necessary, the intravenous route. Patients with clinically significant heart disease may require fluid restriction (2/3) and diuretics.
A third of the patients may have hyponatraemia (<135mEq/L),14 which has shown a stronger association with lobar forms of CAP and higher degrees of severity.14,15 Hyponatraemia is usually due to syndrome of inappropriate secretion of antidiuretic hormone (SIADH), although secretion may be appropriate in hypovolemic children. Similarly, there is evidence of increased atrial natriuretic peptide levels in children with CAP and SIADH. The administration of isotonic fluids is recommended in children admitted with CAP to prevent the development of hyponatraemia.
Respiratory physiotherapyThe available data is scarce. A supported sitting position is recommended to help lung expansion.10
NutritionMalnutrition carries a poorer prognosis. Patients unable to tolerate enteral nutrition may require nasogastric tube feeding.10
CorticosteroidsCorticosteroids seem to shorten the duration of disease in adults.16. Two small trials on children, one on patients with severe CAP and another in patients with CAP caused by Mycoplasma, showed that corticosteroid treatment shortened the duration of disease,17,18 even at high-doses for the treatment of refractory cases.19 Clinical trials are currently being developed to analyse their usefulness in the treatment of CAP and parapneumonic pleural effusion.
Antibiotic therapyAntibiotic therapy in hospitalised children with no underlying diseaseTypical community-acquired pneumonia with a suspected or confirmed pneumococcal aetiologyThe most frequent causative agent in typical forms of CAP continues to be Streptococcus pneumoniae, which at present has an excellent susceptibility profile to beta-lactams such as penicillin, amoxicillin and ampicillin.20 Thus, the first-line antibiotic for the treatment of children older than 3 months with typical CAP that requires hospital admission (Table 1) with a suspected or confirmed pneumococcal aetiology is ampicillin or penicillin G sodium, administered intravenously at high doses, given its excellent tolerability.11 Both choices are equally appropriate, although ampicillin has the advantage of requiring fewer doses a day and having a slightly lower cost. Table 2 shows the dosage for these antibiotics.
Antibiotic treatment of children hospitalised with typical community-acquired pneumonia (with a suspected or confirmed pneumococcal aetiology), depending on the presence of parapneumonic pleural effusion.
| Recommended antibiotic treatment | |
|---|---|
| Without parapneumonic pleural effusion | Equally valid options:– Ampicillin IV: 150–200mg/kg/day, every 6h (maximum 12g/day)– Penicillin G sodium IV: 250000–300000IU/kg/day, every 4h (maximum 24 million IU/day) |
| With parapneumonic pleural effusion | Equally valid options:– Ampicillin IV: 250–300mg/kg/day, every 6h (maximum 12g/day)– Penicillin G sodium IV: 300000–400000IU/kg/day, every 4h (maximum 24 million IU/day) |
This recommendation is mostly based on the expert recommendations documented in clinical guidelines. There have been no clinical trials that compared the efficacy of these antibiotics with that of broader-spectrum antibiotics, although there are retrospective studies that suggest the results are comparable.21
Hospitalised patients usually improve and can be switched to oral amoxicillin when they have remained afebrile for 24–48h. We recommend a course of treatment lasting 7–10 days.
There is a high and unwarranted use of third-generation cephalosporins in hospitalised children with CAP. Thus, a multicentric study conducted in Spain showed that up to 34% of them received initial empirical treatment with these agents.22
Typical community-acquired pneumonia associated with other pathogensTable 3 presents various situations in which treatment with other antibiotics is recommended.
Empirical antibiotic treatment in special cases of typical community-acquired pneumonia, with or without parapneumonic pleural effusion (use the upper limit of the doses in cases with pleural effusion).
| Recommended antibiotic treatment | |
|---|---|
| Younger than 6 months | – Younger than 3 months: ampicillin IV (200mg/kg/day, every 6h)+cefotaxime IV (200mg/kg/day, every 6h)– 3 to 6 months: amoxicillin–clavulanic acid IV (proportion, 10:1): 150mg/kg/day, every 6h |
| Children not vaccinated against Haemophilus infuenzae type b | Options:– Amoxicillin–clavulanic acid IV (proportion, 10:1): 150mg/kg/day, every 6h (maximum 2g every 6h)– Cefuroxime IV: 150mg/kg/day, every 6–8h |
| Suspicion of Streptococcus pyogenesa | Penicillin G sodium IV (250000IU/kg/day, every 4h)+clindamycin IV (30–40mg/kg/day, every 6h) |
| Suspicion of methicillin-susceptible Staphylococcus aureusb | Options:– Cloxacillin IV (150–200mg/kg/day, every 6h)+cefotaxime IV (200mg/kg/day, every 4–6h)– Amoxicillin–clavulanic acid IV (proportion, 10:1): 150mg/kg/day, every 6h (maximum 2g every 6h)– Cefuroxime IV: 150mg/kg/day, every 6–8h |
| Pulmonary abscess and necrotising pneumoniac | Cefotaxime IV (200mg/kg/day, every 6h)+clindamycin IV (30–40mg/kg/day, every 6–8h) |
| Suspected aspiration pneumonia | Amoxicillin–clavulanic acid (proportion, 10:1) IV: 150mg/kg/day, every 6h (maximum 2g every 6h) |
| Allergic to beta-lactam antibiotics | Allergy, not anaphylaxis: cephalosporins, preferably cefuroxime, oral or IVAnaphylaxis:– Mild-moderate CAP: levofloxacin or glycopeptides antibioticsd– Severe CAP: glycopeptides+levofloxacin or macrolides |
Circumstances that suggest the likelihood of S. pyogenes: varicella, pleural fluid negative for pneumococcal antigen; scarlatiniform rash; pharyngeal swab positive for this bacterium; sepsis, poor general health status.
Circumstances that suggest the likelihood of S. aureus: necrotising pneumonia and/or pneumatocoeles; microbiological findings such as pleural fluid negative for pneumococcal antigen, suspicious Gram-positive cocci in pleural fluid, blood culture positive for this bacterium; previous skin or soft-tissue staphylococcal infection; children less than 2–3 years of age that respond poorly to appropriate antibiotic therapy; septic condition; poor general health status.
Amoxicillin/clavulanic acid is recommended in children that have not been vaccinated against Haemophilus influenzae (H. influenzae) type b and children less than 6 months of age, except for children younger than 3 months, in whom the recommended treatment is ampicillin–cefotaxime.
In certain situations, and especially in severe cases, other bacteria should be considered, such as S. aureus and S. pyogenes, requiring different antibiotic treatment (Table 3). The recommended empirical treatment for necrotising forms, which amount to 0.8% of CAP cases (and 6% of hospitalised patients with CAP) and in Spain are usually associated with S. aureus (usually methicillin-susceptible but PVL-producing) followed by S. pneumoniae,5 consists of a cefotaxime and clindamycin regimen for a minimum of 14–21 days.
In children with CAP associated with influenza,10 usually caused by S. pneumoniae or less frequently by S. aureus, S. pyogenes or H. influenzae5,23, empirical treatment with amoxicillin–clavulanic acid as opposed to amoxicillin or ampicillin is recommended.
In children with varicella that have CAP of a suspected bacterial aetiology, the recommended treatment is antibiotics covering S. pyogenes and S. aureus, such as cefuroxime, or in more severe cases, penicillin G or cefotaxime combined with clindamycin, especially in cases of necrotising pneumonia of with signs of toxic shock syndrome.24
Community-acquired pneumonia complicated by parapneumonic pleural effusionThe same antibiotics used in CAP without pleural effusion (ampicillin or penicillin) are recommended (Table 2), save for exceptional cases detailed in Table 3,10,11 although they should be administered at higher doses to achieve adequate pleural fluid concentrations.25
It is recommended that administration is switched to the oral route once the patient has remained afebrile for 48h, for a total course lasting 2–4 weeks depending on the causative agent, although treatment could be extended for a few more days in prolonged or severe cases.
Atypical community-acquired pneumoniaThese cases are most frequently caused by viral infections, especially in children younger than 4 or 5 years, and they do not usually require admission to the hospital or antibiotic treatment. In children older than 4 or 5 years, in whom disease is more frequently caused by Mycoplasma pneumoniae and to a much lesser degree by Chlamydophila pneumoniae, treatment with macrolides is recommended, by the oral route whenever possible and otherwise intravenously.11
Clarithromycin and azithromycin are the two most commonly used antibiotics, at the same doses for both the oral and the intravenous preparations, with azithromycin being better tolerated11 (Table 4). The use of erythromycin has waned due to its adverse effects, including phlebotoxicity when administered intravenously, and its complicated dosage (every 6h for 10–14 days).
Atypical community-acquired pneumonia with confirmation or high suspicion of Mycoplasma or Chlamydophila. Most commonly used macrolides (same dosage for oral and intravenous routes).
| Name | Dosage | Duration |
|---|---|---|
| Azithromycin | 10mg/kg every 24h (maximum dose: 500mg/day)a | 3 days |
| Clarithromycin | 15mg/kg/day, every 12h (maximum dose: 1g/day) | 7 days |
In the United States, the same total dosage is used, but it is distributed over five days (day 1, 10mg/kg, to a maximum of 500mg; days 2–5, 5mg/kg every 24h, to a maximum of 250mg/day), as this is the dosage approved by the FDA, but it does not have any advantages over the 3-day course approved by the European Medicines Agency (EMA).
Influenza can be treated with antivirals, and there are no oseltamivir-resistant strains in Spain. There are reasonable doubts concerning the effectiveness of oseltamivir in hospitalised patients with no risk factors,26 so its use should be restricted to hypoxaemic or seriously ill patients, especially those with significant underlying disease.
Severe community-acquired pneumonia that requires admission to the Paediatric Intensive Care UnitThe aetiological spectrum is broader: S. pneumoniae, S. aureus, S. pyogenes, and others. This consensus recommends cefotaxime, possibly in combination with an antibiotic with antistaphylococcal activity such as cloxacillin (Table 5). Considering the association of S. aureus, including MRSA, and influenza, it would be appropriate to use clindamycin (or vancomycin, depending on local data on susceptibility) combined with a cephalosporin (cefuroxime or cefotaxime).
Recommended antibiotic treatment for children with community-acquired pneumonia and severe pleural effusion that required PICU admission.
| Typical CAP | – Cefotaxime (200–300mg/kg/day, every 6h)+one of the following:a– Cloxacillin IV (150–200mg/kg/day, every 6h)or– Clindamycin IV (30–40mg/kg/day, every 6–8h)b,or– Vancomycin IV (60mg/kg/day, every 6h)c±Macrolide IV (erythromycin 40mg/kg/day every 6h, or clarithromycin 15mg/kg/day, every 12h; or azithromycin 10mg/kg/day, every 24h) |
| Community-acquired interstitial pneumonia | – Cefotaxime (200mg/kg/day)+macrolide IV (erythromycin, clarithromycin or azithromycin)±– Cotrimoxazol IV (20mg of trimetoprim/kg/day every 6h)d |
H, hours; IV, intravenous route; MRSA, methicillin-resistant Staphylococcus aureus; PICU, paediatric intensive care unit.
In adults, combination treatment with a macrolide results in reduced mortality,27 but there are no data on this subject for children. A study of children more than 5 years of age hospitalized with CAP of varying severity found that they had shorter lengths of stay.28
Antibiotic treatment in patients with underlying diseaseAlthough cases of pneumonia in these patients are usually community-acquired, they may have special characteristics: a great variety of potentially involved microorganisms, frequent coinfections, a broad range of clinical findings and a potentially greater severity. These circumstances lead to a greater number of diagnostic tests and more aggressive treatments.
The probable aetiological agents depend on the underlying disease (Table 6) and the characteristics of radiographic findings. Usual pathogens such as respiratory viruses and S. pneumoniae should be generally considered, as well as non-typeable H. influenzae.12
Most common causative agents associated with pneumonia in children with severe underlying disease.a
| Underlying disease | Main aetiological agents | Most commonly used treatments |
|---|---|---|
| Humoral immunodeficiency | S. pneumoniae, H. influenzaeLess common: Salmonella, Pseudomonas, S. aureusEnterovirus | Amoxicillin–clavulanic acid, cefotaxime |
| Combined immunodeficiencyb | – Encapsulated bacteria, Listeria, P. aeruginosa, Stenotrophomonas, Burkholderia cepacia, Legionella, Nocardia– VZV, rubella, VHS, CMV, adenovirus, RSV, influenza, parainfluenza, hMPV– MAC, M. fortuitum, BCG, other opportunistic mycobacteria. Reactivation TB– Pneumocystis jirovecii, Aspergillus, C. albicans, Cryptococcus | Cefotaxime, cefepime, cotrimoxazol, acyclovir, antifungals (fluconazole, voriconazole, liposomal amphotericin B) |
| Phagocytic cell disorders/neutropenia | S. aureus, P. aeruginosa, Stenotrophomonas, B. cepaciaS. marcescens, enterobacteria such as Salmonella, E. colic, Nocardia. BCG, non-tuberculous mycobacteriadAspergillus, Candida | Amoxicillin–clavulanic acid, cefotaxime. Cloxacillin, clindamycin, vancomycin. Cotrimoxazol. Piperacillin–tazobactam, cefepime or meropenem. Antifungals (voriconazole, liposomal amphotericin B) |
| HIV infection | S. pneumoniae, H. influenzae, S. aureus. Mycobacteria. Pneumocystis. CMV, HSV | Amoxicillin–clavulanic acid, cefotaxime. Cotrimoxazol. Acyclovir |
| HSCT | Multiple. Depends on the time of the transplantation, presence of GVHD, IS treatment and antimicrobial prophylaxis. Always encapsulated bacteria | Same as for combined immunodeficiency |
| SOT | Multiple. Depends on the time of the transplantation, IS treatment and antimicrobial prophylaxis. Always encapsulated bacteria | Same as for combined immunodeficiency |
| Complement deficiency | S. pneumoniae, H. influenzae | Cefotaxime |
| Sickle cell disease/asplenia | S. pneumoniae, H. influenzae, Salmonella, and other encapsulated bacteria | Cefotaxime |
| Nephrotic syndromee | S. pneumoniae, H. influenzae, Enterobacteria. Respiratory viruses | Amoxicillin–clavulanic acid, cefuroxime or cefotaxime |
| Rheumatic or anti-inflammatory diseasee | S. pneumoniae, H. influenzae and other encapsulated bacteria | Amoxicillin–clavulanic acid, cefuroxime or cefotaxime. Levofloxacin |
| Cystic fibrosisf | P. aeruginosa, S. aureus, Burkholderia, Stenotrophomonas. H. influenzae. Aspergillus (allergic bronchopulmonary aspergillosis) | Amoxicillin–clavulanic acidgCeftazidime, piperacillin–tazobactam, meropenem, plus an aminoglycoside, or ciprofloxacin. Cloxacillin |
| Cardiac disease | Common pathogens. Respiratory viruses | Amoxicillin–clavulanic acid, cefuroxime or cefotaxime |
BCG, bacillus Calmette-Guérin; CMV, cytomegalovirus; GVHD, graft-versus-host disease; HIV, human immunodeficiency virus; hMPV, human metapneumovirus; HSCT, haematopoietic stem cell transplantation; HSV, herpes simplex virus; IS, immunosuppressive; MAC, Mycobacterium avium complex; RSV, respiratory syncytial virus; SOT, solid organ transplantation; TB, tuberculosis; VZV, varicella zoster virus.
Organisms that cause CAP in healthy children should always be considered, especially respiratory viruses and S. pneumoniae, and to a lesser degree H. influenzae. The degree of immunosuppression, the immediate history (recent hospitalisation, chemoprophylaxis, recent vaccinations) and the clinical condition should guide the clinician in deciding how aggressive an approach to take to the management of the patient (need for admission, invasive diagnostic and microbiological tests, combination antibiotic treatment, use of antivirals and antifungals).
Suppression of cell-mediated immunity could lead to hyperinfection by Strongyloides stercoralis (rule out in cases of severe eosinophilia; it is important to known the country of origin of the patient).
It is important to consider endemic infections in the country of origin; there is evidence that the incidence of TB in adults with blood cancers is very high in the immigrant population.
Especially important in IF-γ and IL-12 receptor deficiency, and Salmonella and Listeria could also be involved.
Depends to a great extent on the immunosuppressive treatment given to the patient,, who may develop infections by opportunistic pathogens. For example, the administration of anti-TNF drugs and corticosteroids at immunosuppressive doses has been associated with tuberculosis reactivation and infection by Cryptococcus, Aspergillus, Listeria, Pneumocystis, HSV, VZV and CMV, among others.
It is important to know the previous history of respiratory tract colonisation. A combination of two antibiotics at higher-than-usual doses is recommended. If the patient is colonised by S. aureus, this pathogen should always be covered. A saliva sample must always be collected before initiating antibiotherapy.
In patients that are not severely immunosuppressed, most of the panel would recommend initiating empiric treatment with amoxicillin–clavulanic acid, cefuroxime or cefotaxime, with the possibility of adding a neuraminidase inhibitor (oseltamivir) if influenza virus is detected. In patients with a higher degree of immunosuppression, a macrolide should be added if there are diffuse pulmonary infiltrates, and even cotrimoxazol if Pneumocystis pneumonia is suspected.
Treatment failureIt is defined as the development of respiratory failure or the persistence of tachypnoea 72h after the onset of disease, or as persistence of fever or of poor general health status 48–72h following admission.10 However, in the latter case, if the patient shows improvement and the levels of acute phase reactants (especially of C reactive protein) decrease, antibiotic treatment has probably not failed.
If treatment is considered to have failed 48–72h after initiation, a clinical, radiological and laboratory re-evaluation should be performed, assessing for the most frequent causes of failure (Table 7).10,29
Most frequent causes of treatment failure in community-acquired pneumonia.
| 1. Pleural effusion, necrotising pneumonia or pulmonary abscess2. Non-susceptible microorganism, most commonly viral. The possibility of initiating macrolide treatment can be considered if Mycoplasma is suspected. It can also be the first sign of tuberculosis3. Non-compliance with treatment or insufficient dose4. Underlying condition in the patient, such as immunosuppression, malnutrition, asthma or cystic fibrosis5. Alternative diagnosis, such as foreign body aspiration, lung malformation or diaphragmatic hernia |
Approximately 20–40% of children admitted with CAP will have pleural effusion, and up to 0.6% will progress to empyema.30
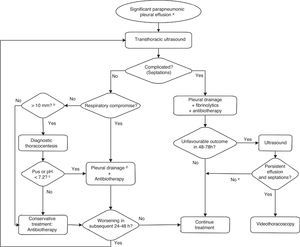
About half of the cases of parapneumonic pleural effusion resolve with antibiotic treatment and do not require invasive interventions.30 The patient's clinical condition (especially breathing difficulty) and the extent of the effusion are key in decision-making. Fig. 1 presents the recommended care protocol for parapneumonic pleural effusion.
Algorithm for the management of significant parapneumonic pleural effusion. (a) Subclinical pleural effusion (costophrenic angle blunting, minimal volume) will be managed as an uncomplicated pneumonia. (b) In selected cases, a diagnostic thoracocentesis may be performed in cases of small effusion. (c) These findings, which are available quickly, are an indication for immediate pleural drainage during the same procedure. Other findings in the pleural fluid that become available at a later time, such as visualisation of bacteria by Gram staining, positive culture, LDH>1000IU/mL, glucose<40–60mg/dL, may be indications for pleural drainage a posteriori. The use of these biochemical data for the purposes of decision-making is outdated. A positive PCR result for a bacterium or a positive S. pneumoniae antigen test (BinaxNow) in pleural fluid will not be used as the sole criteria for chest tube placement. (d) In the absence of empyema, under certain circumstances and in certain patients, if staff trained in the placement and maintenance of a chest drain were not available promptly, a therapeutic thoracocentesis could be performed. (e) Consider other causes for poor outcome: necrotising pneumonia, pulmonary abscess.
Whenever there is a moderate to large pleural effusion or there is doubt as to the presence or extent of a small effusion in the radiograph, a thoracic ultrasound should be performed. The extent and sonographic characteristics of the effusion should be determined, along with the optimal puncture site or sites for a potential chest drain, searching for the thickest area that can be accessed most easily, which is usually found between the fifth and the seventh intercostal spaces at the level of the mid-posterior axillary line. Drainage should not be performed in cases of subpulmonic effusion. Effusions smaller than 10mm should not be performed routinely.11
Thoracocentesis is to be performed in a properly equipped room (anaesthesia, PICU, or operating room), although there may be other equally fitted areas in the emergency department or inpatient wards. We recommend that chest tube insertion be performed in an intensive care unit or operating room.
A chest tube should be placed if the effusion meets one or more of the criteria for pleural empyema or if it causes moderate to severe breathing difficulty. A complicated effusion with septations requires not only tube drainage but also administration of fibrinolytics, and if the patient does not respond favourably, performance of video-assisted thoracoscopic surgery (VATS).
Catheters of variable size may be used. The American Pediatric Surgical Association recommends using 12F catheters.31 A recent multicentric study in Spain used 12F–14F catheters,32 although other studies have used 8F–10F tubes.33 Small-bore catheters (8F–14F) are as effective as large-bore catheters, cause less pain, and shorten the length of stay.34 The use of trocars is discouraged, and it is recommended that soft tubes are used for insertion with the Seldinger technique. Radiography should be used to confirm proper placement of the tube and rule out pneumothorax. The tube has to be connected to a unidirectional flow drainage system that must be kept at a level lower than the patient's chest. The indications for using a drainage system remain unclear, but it seems to improve fluid removal. When these systems are used, a water seal must be used at a pressure of 5–10cm H2O.
The drain should be clamped for 1h once 10mL/kg have been removed. In older children and adolescents, it is recommended that no more than 1.5L of fluid are removed at one time, or that larger amounts are drained slowly at a rate of approximately 500mL per hour.
The drain is usually removed when there is minimal drainage (<40–60mL/24h).32,33 Some authors recommend keeping the drain until serous fluid drainage amounts to less than 1mL/kg/day for the previous 12h. The absence of a significant amount of fluid in ultrasound examination may guide this decision.11 Drain removal does not require previous clamping.
FibrinolyticsWe propose the use of fibrinolytics for the first-line treatment of pleural effusion with septations, either with floating septa or forming loculations (Fig. 1).31,32 Their use has proven to be cost-effective compared to placement of chest drains without fibrinolytics.35 In cases with no sonographic septations but with a lower than expected drainage volume, and especially if the fluid is thick, treatment with fibrinolytics should be considered once other possible causes have been ruled out.
Out of all the available fibrinolytic agents, this panel recommends the use of urokinase because it is the one for which there is the most data for the paediatric age group.
Clinical trials comparing intrapleural urokinase with VATS in children32,33 found no differences between these two interventions. A recent multicentre clinical trial conducted in Spain compared the usefulness of urokinase and VATS in 103 children with fibrinopurulent pleural effusion.32 The results showed that urokinase is as effective as VATS in the treatment of septated pleural empyema, while no differences were found in the length of stay from the time treatment was initiated.32
These results support the current recommendation of the American Pediatric Surgical Association of using fibrinolytics as the first-line treatment of these patients, given its lower cost and easier implementation, as it does not require surgical intervention.31–33,36
The urokinase doses used in different studies range between 10000 and 100000IU.37 The recommendation in this consensus is the following:32
- -
Infants age<1 year: 10000IU in 10mL of 0.9% saline solution.
- -
Children age≥1 year: 40000IU in 40mL of 0.9% saline solution.
It is administered through the intrapleural catheter (which is subsequently clamped for 4h), twice a day for 3 days.32,33 Additional doses can be used if the patient has not fully responded after the initial six.37
The administration of fibrinolytics through a chest tube may cause discomfort, so it must be accompanied by appropriate analgesia. It may also cause light bleeding and, on rare occasions, immediate hypersensitivity reactions.
Fibrinolytic therapy should be discontinued if it is ineffective, which can happen in highly organised effusions, and should not be considered in patients with bronchopleural fistula when bubbling is observed in the chest tube, as it is suggestive of an air leak. In the latter case, clamping the tube could cause tension pneumothorax. Chest drains should be unclamped immediately if the child develops signs of clinical worsening, such as increased breathing difficulty or chest pain.
Video-assisted thoracoscopic surgeryVideo-assisted thoracoscopic surgery can be used to determine the stage of the effusion, break the septations, drain the fibrinopurulent material, reduce the bacterial load in the initial stages, and place the chest drain correctly. Furthermore, it allows the visualisation of the underlying lung, its expansion capacity, and the location of bronchopleural fistulae.
At present, there are two accepted indications for VATS:
- -
Persistent moderate to massive effusion with respiratory compromise despite treatment with drainage and fibrinolytics for 2–3 days,11 which usually happens in 15% of cases.33,36
- -
Complications such as bronchopleural fistula.33
It is also an option for the initial treatment of highly fibrinopurulent and organised effusions in which a thick fibrous peel has developed, or when treatment with fibrinolytics cannot be implemented.31,38 Its use as initial treatment must be considered on a case-to-case basis, taking into account the duration and characteristics of the effusion, as well as the availability and surgical experience of the centre for performing VATS. Decortication is not usually necessary in children.
The efficacy of VATS for the treatment of empyema is quite high. Compared to plain drainage without fibrinolysis, VATS significantly reduces the duration of symptoms (fever usually resolves in 24–72h), the length of stay (reduced to 6–7 days) and the cost of healthcare.31,39 On the other hand, its efficacy seems to be similar to that of treatment with drainage and fibrinolytics.32,33,36,40 It is estimated that the cost of VATS is higher than that of fibrinolytic treatment,33,35,36 although some authors believe that performing VATS early on could be more efficacious and reduce healthcare costs.38
The rate of complications is low (6–7%), including air leaks of persistent pneumothorax, pneumatocele, or bleeding.38
Conflicts of interestConflicts of interest of the authors in relation to this document (past five years):
- -
DMP has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, as a researcher in a clinical trial for Novartis, and as a consultant in the Advisory Boards of Astra-Zeneca and Pfizer.
- -
AAM has no conflicts of interest to declare.
- -
ATG has collaborated in research activities funded by Pfizer.
- -
AEM has collaborated in educational activities funded by Novartis, as a researcher in a multicentre study promoted by GlaxoSmithKline and as a consultant in an Advisory Board for Gilead.
- -
JFM has collaborated in educational activities funded by Gilead and Abbvie.
- -
JGG has collaborated in educational activities funded by Pfizer and Sanofi Pasteur MSD.
- -
AMG has participated as a consultant in the Advisory Boards of Abbvie and Gilead, has received institutional research grants from Abbvie and grants for attending scientific conferences from Abbvie, Actelion, Ferrer, GlaxoSmithKline and Novartis.
- -
CRGL has collaborated in educational activities funded by GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD, as a researcher for clinical trials for GlaxoSmithKline and a consultant in the Advisory Boards of Astra-Zeneca, Novartis, GlaxoSmithKline and Pfizer.
- -
JSL has collaborated as a researcher in clinical trials for GlaxoSmithKline and Roche.
Moreno-Pérez D [Grupo de Trabajo de Infecciones Respiratorias. Sociedad Española de Infectología Pediátrica (SEIP)], Andrés Martín A [Sociedad Española de Neumología Pediátrica (SENP)], Tagarro García A, Escribano Montaner A [Sociedad Española de Neumología Pediátrica (SENP)], Figuerola Mulet J [Sociedad Española de Neumología Pediátrica (SENP)], García García JJ [Grupo de Trabajo de Infecciones Respiratorias. Sociedad Española de Infectología Pediátrica (SEIP)], Moreno-Galdó A [Sociedad Española de Neumología Pediátrica (SENP)], Rodrigo Gonzalo de Lliria C [Grupo de Trabajo de Infecciones Respiratorias. Sociedad Española de Infectología Pediátrica (SEIP)], Saavedra Lozano J [Grupo de Trabajo de Infecciones Respiratorias. Sociedad Española de Infectología Pediátrica (SEIP)].
Please cite this article as: Moreno-Pérez D, Andrés Martín A, Tagarro García A, Escribano Montaner A, Figuerola Mulet J, García García JJ, et al. Neumonía adquirida en la comunidad: tratamiento de los casos complicados y en situaciones especiales. Documento de consenso de la Sociedad Española de Infectología Pediátrica (SEIP) y Sociedad Española de Neumología Pediátrica (SENP). An Pediatr (Barc). 2015;83:226.