Antibiotherapy regimens for management of acute streptococcal pharyngitis traditionally last 10 days, but the development of resistance to different antimicrobials has motivated the exploration of shorter courses.
Material and methodsWe selected patients given a diagnosis of streptococcal pharyngitis in 2 paediatric caseloads of 1 primary care centre between June 2016 and April 2020. We compared outcomes in patients treated with 8- to 10-day courses versus 5- to 7-day courses.
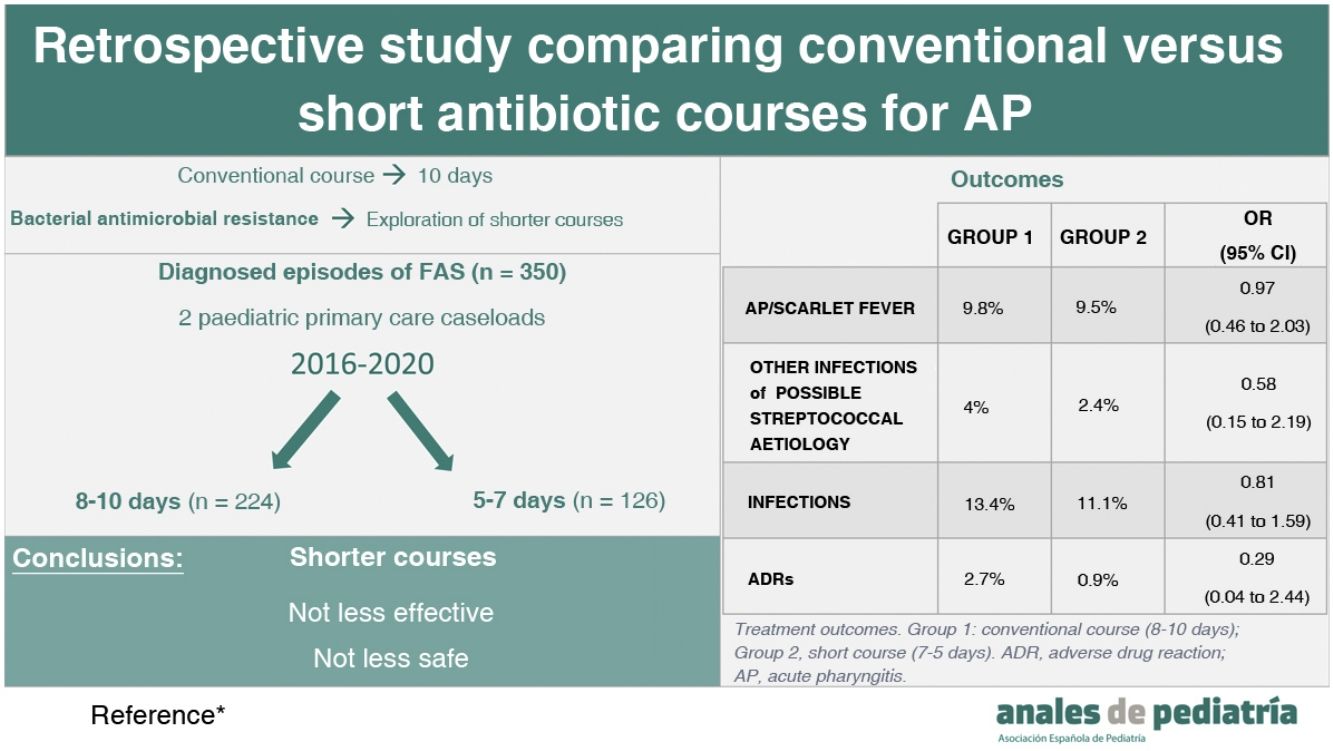
ResultsThe analysis included 350 care episodes (252 patients). Sixty-four percent were managed with 8- to 10-day courses of antibiotherapy (group 1) and 36% with 5- to 7-day courses (group 2). There were no significant differences in the incidence of streptococcal pharyngitis or scarlet fever in the 3 months that followed (OR, 0.98; 95% confidence interval [CI], 0.46–2.03), with similar percentages in both groups (9.8% vs 9.5%). Overall, without differentiating based on the type of infection (streptococcal pharyngitis, scarlet fever or other streptococcal infections), we found similar outcomes (OR, 0.81; 95% CI, 0.41–1.59): 13.4% in group 1 and 11.1% in group 2. We also found no differences in the frequency of adverse events documented in the health records (OR, 0.29; 95% CI, 0.04–2.44): 2.7% in group 1 and 0.8% in group 2.
ConclusionsIn our experience, a shorter antibiotic course (5–7 days) is not less effective or more unsafe for management of acute streptococcal pharyngitis than the traditional 10-day course.
El tratamiento antibiótico de la faringoamigdalitis aguda estreptocócica (FAS) clásicamente es una pauta de 10 días; sin embargo, la aparición de resistencias antibióticas induce a explorar pautas más cortas.
Material y métodosSeleccionamos aquellos pacientes diagnosticados de FAS en 2 cupos de pediatría de un Centro de Salud entre junio de 2016 y abril de 2020. Se compararon los resultados de aquellos que recibieron tratamiento 8–10 días y aquellos que recibieron 5–7 días.
ResultadosSe analizaron 350 episodios (252 pacientes). El 64% recibieron tratamiento durante 10-8 días (grupo 1) y el 36% durante 7–5 días (grupo 2). No se observaron diferencias significativas en la aparición de FAS o escarlatina los 3 meses posteriores (OR 0,97; IC 95%: 0,46–2,03) siendo similar la proporción en ambos grupos (9,8% vs 9,5%). Sin diferenciar el tipo de infección (FAS, escarlatina u otro tipo de infección streptocócica) se observaron resultados similares (OR 0,81; IC 95%: 0,41–1,59) con un 13,4% en el grupo 1 y 11,1% en el 2. Respecto a la aparición de reacciones adversas medicamentosas recogidas en la historia clínica fue de 2,7% en el grupo 1 y 0,8% en el 2 (OR 0,29; IC 95%: 0,04–2,44).
ConclusionesSegún nuestra experiencia la pauta antibiótica corta (5–7 días) en faringoamigdalitis aguda estreptocócica no es menos efectiva ni más insegura que la clásica pauta de 10 días.
Acute pharyngitis (AP) is a common disease in childhood, and actually one of the most frequent reasons for paediatric primary care visits. It has a viral aetiology in 75% to 80% of cases, and, in the cases with a bacterial aetiology, streptococcus is the most frequent causative agent (30%–40%).
The antibiotic of choice for treatment of AP is penicillin V, administered orally, although amoxicillin is a preferred alternative in many cases on account of its palatability and not interfering with eating, which facilitates its administration in children. The goal of treatment is to accelerate recovery and prevent complications, be it suppurative, like tonsillar abscess, or non-suppurative, like the classic acute rheumatic fever presentation. It also aims to prevent transmission and the development of outbreaks. While antibiotherapy may be indicated, it is worth noting that untreated cases tend to resolve spontaneously within 5 days without complications, and that the prevalence of rheumatic fever in Spain is minimal.
The 10-day antibiotherapy course was established in 1958 based on the early studies conducted in the United States, and its main goal was bacterial eradication, as it was believed that this reduced the likelihood of acute rheumatic fever, which was always the main indication of treatment.1 However, the infectivity of individuals with pharyngeal carriage, and therefore the real need to achieve eradication, remain unknown; at present, it is hypothesised that these carriers rarely transmit the microorganism and therefore pose a low risk to contacts.2
Few studies in the literature have compared the 10-day antibiotherapy course for AP to shorter courses. Studies that have focused on clinical outcomes (incidence of complications or cure rate) have not found statistically significant differences.3–5 However, studies that have maintained the goal of bacterial eradication found a decreased success rate with the short course of treatment.6 Thus, some authors are now proposing the use of short courses of treatment unless bacterial eradication is specifically pursued.7
The drive to use shorter courses mainly stems from the emergence of antibiotic resistance. Although resistance to beta-lactam antibiotics has yet to be described in Streptococcus pyogenes, it is important to keep in mind that whenever antibiotherapy is used, all the bacteria in the organism experience a pressure that can result in the selection of those that are drug resistant. One example in clinical practice is found in the treatment of Escherichia coli, one of the microorganisms most frequently involved in urinary tract infections in children with a prevalence of amoxicillin resistance in our area of 56% (data for 2019 provided by the reference microbiology laboratory of our primary care centre).
There are other benefits, too, as shorter courses of antibiotherapy have been found to be associated with fewer complications, greater adherence to treatment and lower costs.8
The aim of our study was to identify potential differences in the rate of reinfection, development of other infections of possible streptococcal aetiology or the incidence of adverse drug reactions based on the use of short or long courses of antibiotherapy for treatment of AP.
Material and methodsStudy designWe conducted a retrospective observational descriptive and analytical study based on the review of health records of patients given a diagnosis of AP in 2 paediatrics caseloads in the José Ramón Muñoz Fernández Primary Care Centre in Zaragoza (Spain), between 2016 and April 2020.
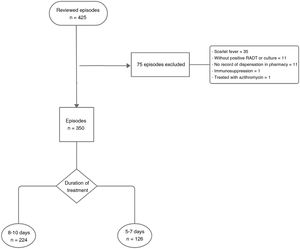
The sample consisted of patients diagnosed of AP based on positive results of a rapid antigen detection test or a throat swab culture. The exclusion criteria were: diagnosis of AP without testing, absence of records of the dispensation of the prescribed antibiotic in the pharmacy management system, scarlet fever and immunosuppression due to illness or medication. We excluded 75 episodes, so the final sample size was of 350 episodes (Fig. 1).
The project was approved by the Research Ethics Committee of Aragón (file PI20/519; 02/12/2020), as it fulfilled the required criteria. The data were collected in a database and anonymised. Since the study was retrospective and received the necessary authorizations, it was not necessary to seek informed consent.
Data collectionAfter the sample selection, we reviewed the electronic health records of the patients in the primary care system and in the larger health care system of the region of Aragón. We collected epidemiological data (sex, age and date of diagnosis) and data on the prescribed antibiotherapy course (antibiotic, days of treatment and, if a change was required, the second prescribed antibiotic). As regards outcomes, we collected information on the development of AP or scarlet fever in the 3 months following the episode under study, the development of other infections of probable streptococcal aetiology (pneumonia acute otitis media, sinusitis, scarlet fever, streptococcal perineal disease and septic arthritis) in the 3 months following the episode, in addition to the type of infection, and any adverse drug reactions documented in the health records.
Statistical analysisIn the comparative analysis, we calculated odds ratios (ORs) and mean differences with the corresponding 95% confidence intervals (CIs). In addition, to assess whether there were any significant differences, and based on whether or not the applicable assumptions were met, we used the χ2 and the Student t test, using the statistical software SPSS version 25.0, and considering P values of less than 0.05 statistically significant.
ResultsThe sample included 350 episodes of AP in 252 patients, as 98 patients (38%) experienced more than 1 episode of AP during the study period.
We divided the sample in 2 groups based on the prescribed duration of treatment. The mean duration was 8.85 days (range, 5–10 días). Episodes in group 1 (long course) were those in which the prescribed duration of treatment was 8 to 10 days and amounted to 64% of the sample (n = 224); while group 2 (short course), consisting of the remaining 36% of episodes (n = 126), corresponded to prescriptions of courses of antibiotherapy for a duration of 5–7 days.
The study period ranged from June 2016 to April 2020, and the distribution of episodes during this period is shown in Fig. 2.
We did not find significant differences between groups in the demographic characteristics of the patients (sex and age) (Table 1).
Demographic characteristics of the patients and antibiotherapy.
| Group 1 | Group 2 | P | ||||
|---|---|---|---|---|---|---|
| n | %/mean ± SD | n | %/mean ± SD | |||
| Sex | Female | 117 | 52% | 58 | 46% | .265 |
| Male | 107 | 48% | 68 | 54% | ||
| Age | 7.26 ± 2.7 | 7.22 ± 2.8 | 0.9 | |||
| Antibiotherapy | Amoxicillin | 178 | 79% | 98 | 78% | .26 |
| Penicillin | 35 | 16% | 27 | 21% | .08 | |
| Other | 11 | 5% | 1 | 0.8% | .055 | |
Group 1: conventional course (8–10 days); Group 2: short course (7–5 days). Other antibiotics: josamycin, clindamycin, cefadroxil and cefuroxime.
The overall seasonal distribution, not differentiating by type of course prescribed, was: 40% of episodes (n = 139) in spring, 9% (n = 32) in summer, 27% (n = 96) in autumn and 24% (n = 83) in winter. We ought to highlight that there were significant differences in incidence between the groups in the spring (P = .02) and winter (P = .006).
Amoxicillin was most frequently prescribed antibiotic in both groups, followed by penicillin. Other antibiotics, which included josamycin, clindamycin, cefadroxil and cefuroxime, were prescribed in a minority of episodes (Table 1). We did not find significant differences in the prescribed antibiotic agents between groups. Two percent of patients in group 1 (n = 5) required switching to a second antibiotic agent, something that did not occur in any patient prescribed a short course of treatment.
As regards the outcomes of treatment (Table 2), 9.8% (n = 22) of group 1 vs 9.5% (n = 12) of group 2 developed a microbiologically confirmed episode of AP or scarlet fever within 3 months of the initial episode (OR, 0.97; 95% CI, 0.46–2.03). We studied patients that had developed scarlet fever separately, and also found no significant differences between groups (OR, 0.7; 95% CI, 0.14–3.4). There were also no significant differences in other infections of possible streptococcal origin: 4% (n = 9) in group 1 and 2.4% in group 2 (n = 3) (OR, 0.58; 95% CI, 0.15–2.19). In group 2, the only documented infection was acute otitis media; in group 1, the documented infections were: acute otitis media (n = 6), sinusitis (n = 1), pneumonia (n = 1) and adenitis (n = 1). Finally, we analysed this outcome independently of the type of infection (AP, scarlet fever or other), and found no significant differences.: 13.4% of group 1 (n = 30) versus 11.1% of group 2 (n = 14), (OR, 0.81; 95% CI, 0.41–1.59).
Treatment outcomes.
| Group 1 | Group 2 | OR (95% CI) | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| AP/scarlet fever | 22 | 9.8% | 12 | 9.5% | 0.97 (0.46 to 2.03) |
| Other infections of possible streptococcal aetiology | 9 | 4% | 3 | 2.4% | 0.58 (0.15 to 2.19) |
| Infections (AP/scarlet fever/other) | 30 | 13.4% | 14 | 11.1% | 0.81 (0.41 to 1.59) |
| ADRs | 6 | 2.7% | 1 | 0.9% | 0.29 (0.04 to 2.44) |
Group 1: conventional course (8–10 days); Group 2, short course (7–5 days). ADR, adverse drug reaction; AP, acute pharyngitis; CI, confidence interval; OR, odds ratio.
Last of all, we analysed the adverse drug reactions documented in the health records, with an incidence of 2.7% (n = 6) in group 1 versus 0.8% (n = 1) in group 2. The documented ADEs were rashes (n = 4) and gastrointestinal complaints (n = 3) (OR, 0.29; 95% CI, 0.04–2.44). We ought to highlight that these adverse reactions occurred in the 2 patients treated with clindamycin.
DiscussionThe demographic characteristics of the patients in this series (age and sex) were similar to those described in the previous literature. As regards seasonality, the observed differences in incidence between sprig and winter may be due to the fact that most of the patients included in group 2 (short course) were diagnosed from spring 2018 onward, a period that includes the lockdown imposed due to the SARS-CoV-2 pandemic (March and April 2020), when there was a general decrease in all respiratory infections.
The most frequently prescribed antibiotics in the sample were amoxicillin and penicillin, in adherence with the recommendations of the most recent international guidelines.7,9 The duration prescribed in most cases was 10 days, based on classical recommendations; from 2018 (which marked approximately the halfway point of the study period), and coinciding with the publication of the NICE guideline,7 shorter courses of antibiotherapy started to be prescribed at our primary care centre.
Few studies have compared the effectiveness and safety of short and long courses of treatment for AP since the long course approach was established in 1958.
In our study, we did not find significant differences between the 2 courses in the development of adverse drug reactions or of additional infections of possible streptococcal aetiology in the 3 months after diagnosis. Yet, in every outcome under study, the observed proportion was greater in group 1.
In 2018, Oliveira et al. published a study on the effectiveness of short antibiotherapy courses conducted in a paediatric emergency department. The results were similar to ours, as they did not find significant differences in the development of complications.3 Skoog et al. carried out a randomised controlled study in which 66% of the sample reported preferring the 5-day course; the authors also analysed the effectiveness and safety of the course, with results that were similar to ours.4
In most studies that compare short and long courses, the primary endpoint has been microbiological eradication. These have indeed found the 10-course to be superior. For instance, the aforementioned trial by Skoog et al. found a lower frequency of eradication with the short course (80.4% vs 90.7%). A meta-analysis conducted by Falagas et al. also found a lower rate of eradication (OR, 0.49; 95% CI, 0.32–0.74) and a higher frequency of therapeutic failure with the short course (OR, 0.49; 95% CI, 0.25–0.96), although the review was not exclusively of paediatric studies and compared heterogeneous regimens.6
In 1997, Gunnarson et al. analysed the frequency of S. pyogenes in healthy children and found a prevalence of 2% to 20% depending on age.10 Subsequent studies conducted in different areas of the world have found similar prevalences.11,12 While microbiological eradication is the primary endpoint in most studies comparing short and long courses of antibiotherapy, there are no studies analysing the role played by carriers or their infectivity.
The objective of the 10-day course is chiefly to prevent suppurative complications and rheumatic fever. The resolution of the disease and clinical improvement are also among its goals, although it is already known that these goals do not require a long course of treatment, as patients with AP tend to improve in the first 24 to 48 h of treatment.
In our clinical experience, suppurative complications, when they occur, usually appear at the time of diagnosis or within a few days, so a 10-day course offers no advantages in this regard.13 When it came to subsequent suppurative infections, our study did not evince significant differences between the short and long courses.
Preventing rheumatic fever was one of the rationales in support of the 10-day course approach, based on studies from the mid-20th century.14 At present, the disease is nearly absent in Europe: there are only sporadic cases in the immigrant population.15 After the initial studies, there have been no others comparing long- and short-course antibiotherapy regimens for treatment of FAS in which the development of rheumatic fever was the primary outcome. Most assume the association between bacterial eradication and rheumatic fever not developing. In 2013, in an analysis of 151 cases of acute rheumatic fever in Australia, Noonan et al. found that only 33% had a previous history of sore throat.16 Thus, the authors proposed that strategies for the prevention of acute rheumatic fever should not be based exclusively on the antibiotic treatment of AP.
Since the mid-20th century, the use of antibiotics has spread worldwide. Just as it became one of the crucial tools in medicine, it has also given rise to what is and will continue to be one of the greatest problems in public health: bacterial drug resistance. This has motivated a reassessment of the indications, dosage and duration of treatments.17 In Spain, one of the strategic lines of the National Plan to Reduce the Selection and Dissemination of Antimicrobial Resistance is the revision of the dosage of treatments that have not yet been updated.18 Guidelines in other countries also recommend the use of the shortest course known to be effective, which would minimise the exposure of the pathogen and the rest of the microbiota to the drug and therefore the selection of resistant strains.7,9,19
Besides minimising exposure, shorter courses offer added benefits, such as a lower incidence of adverse drug reactions, greater adherence to treatment5,20 and reduced costs.
In light of all of the above, the most recent guidelines have started to recommend courses lasting 5–7 days, except in patients with risk factors or in circumstances in which eradication is a priority.5,7,9
One of the main limitations of our study, besides those intrinsic to the retrospective design with collection of data from health records, is that we obtained information on the prescribed duration of treatment, but were unable to verify the actual administration of antibiotherapy or exact duration of treatment in days. To reduce this source of information bias, we only included patients for whom we found dispensation records.
Since the rates of bacterial drug resistance have to be brought down, we believe that this type of studies, whose ultimate aim is the optimisation of antimicrobial prescribing, need to be conducted. Clinical trials should be performed to establish the precise duration of treatment with beta-lactam antibiotics required for paediatric AP and thus provide a solid and definitive basis for the necessary reduction in the duration of treatment. Another aspect that also remains to be addressed is the accurate assessment of the impact of carriage and the associate infectivity on both colonised patients and their environment.
FundingThis research did not receive any external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Ana Belén Salinas Bastarras, statistician.