In neonatal resuscitation, it is important to know whether the use of a combination of quality assessment tools has an impact on the preparation of the resuscitation bed and equipment, the correct performance of the procedure and the clinical outcomes of the most vulnerable neonates.
Material and methodsMulticentre, prospective, quasi-experimental interventional study in five level III-A neonatal units. In the pre- and post-intervention phases, both of which lasted 1 year, there were weekly random audits of the stabilization beds in the delivery room to assess their preparation. In the post-intervention phase, checklists, briefings and debriefings were used in the resuscitation of neonates delivered before 32 weeks. We compared the performance of the procedure and early post-resuscitation outcomes in the 2 periods.
ResultsTotal of 852 audits were carried out in the pre-intervention period and 877 in the post-intervention period. There was a greater percentage of audits that did not identify defects in the second phase (63% vs 81%; P < .001). The first phase included 75 resuscitations and the second 48, out of which all the quality assessment tools had been used in 36 (75%). We did not find any differences in the main clinical variables during stabilization, although we observed a trend towards fewer technical problems during the procedure in the second period.
ConclusionsThe use of random audits, checklists, briefings and debriefings in the resuscitation of newborns delivered before 32 weeks is feasible but has no impact on short-term clinical outcomes or correct performance of the procedure. Audits of neonatal resuscitation beds significantly improved their preparation.
Es importante conocer si, en la reanimación neonatal, el uso de diversas herramientas de calidad tiene impacto en la preparación del puesto de estabilización, correcto desarrollo del procedimiento y evolución clínica de aquellos neonatos más vulnerables.
Material y métodosEstudio de intervención cuasiexperimental, prospectivo y multicéntrico en cinco Unidades Neonatales III-A. En las fases pre y posintervención, ambas de un año de duración, se realizaron auditorías aleatorias semanales de los puestos de estabilización en paritorio para comprobar su preparación. En la fase posintervención se usaron checklists, briefings y debriefings en las reanimaciones de los neonatos menores de 32 semanas. Se compararon el desarrollo del procedimiento y la evolución inicial posreanimación entre ambos periodos.
ResultadosSe realizaron 852 auditorías en el periodo preintervención y 877 en el posintervención. El porcentaje de auditorías sin defecto fue superior en la segunda fase (63% vs 81% p < 0,001). Se incluyeron 75 reanimaciones en la fase inicial y 48 en la segunda, de las cuales en 36 (75%) se habían utilizado todas las herramientas de calidad. No existieron diferencias en las principales variables clínicas durante la estabilización, aunque se objetivó una tendencia a menores problemas técnicos durante el procedimiento en el segundo periodo.
ConclusionesLa utilización de auditorías aleatorias, checklists, briefings y debriefings en la reanimación de los menores de 32 semanas es factible pero no tiene impacto en los resultados clínicos a corto plazo ni en la correcta ejecución del procedimiento. Las auditorías de los puestos de reanimación neonatal mejoran significativamente su preparación.
Adverse events due to unsafe care are an important cause of morbidity and mortality, and different studies have evinced that their frequency and severity may be greater in newborns compared to adult inpatients.1–3 When it comes to neonatal cardiopulmonary resuscitation (CPR), as is the case in any other procedure, there are also unsafe care incidents that can have potentially severe consequences, especially in the most immature infants.4–6 To minimise these incidents and improve care delivery, a variety of tools have been developed to assess and/or improve health care quality, such as checklists or brief meetings before and after CPR delivery (briefings and debriefings). These tools can improve readiness and adherence to the initial steps of neonatal CPR, in addition to teamwork and communication in the resuscitation team.7,8 Recently, the Sociedad Española de Neonatología (SENeo, Spanish Society of Neonatology) recommended holding briefings before delivery of neonatal CPR as well as debriefings after the intervention.9
Real-time random safety audits (RTRSAs) are another safety tool that enables the detection of errors and potentially harmful care practices. In this approach, a checklist is randomly applied to assess certain equipment or a given procedure in real time to identify weak points in care safety. These audits are inexpensive and easy to do. Their application in the neonatal care setting is still limited.10–14
Given the importance of delivering safe and high-quality neonatal CPR and the need to improve morbidity in the most immature infants, we conducted a study to determine the clinical impact of the introduction of RTRSAs in neonatal stabilization areas combined with the use of checklists, briefings and debriefings in newborn resuscitation procedures in neonates born before 32 weeks of gestational age (<32 wGA) in several tertiary care neonatal units in our region.
Material and methodsWe conducted a multicentre, prospective quasi-experimental interventional study in 5 neonatal intensive care units (NICUs) in the Community of Madrid, Spain, all of them classified as Level IIIA based on the criteria established by the SENeo.15 Before its initiation, the study was approved by the ethics committees of each of the participating institutions.
For one year, weekly RTRSAs were performed in at least 3 neonatal stabilization areas per centre out of the total of 36 areas available in the 5 labour and delivery units (preintervention phase, October 2018–September 2019). Each audit consisted of a thorough examination of the setup of the resuscitation bed and the equipment, supplies and drugs needed for neonatal stabilization (detailed in supplementary material, Appendix B). The correct preparation and stocking of each area was assessed using the recommendations of the Neonatal Resuscitation Group of the SENeo as reference.16 The audit yielded an overall result for the area: no errors (correct preparation), minor errors (easy to detect and fix, even at the time of resuscitation, and unlikely to cause adverse events in the newborn) or serious errors (any not easy to detect or fix that could result in serious adverse events during the procedure). The RTRSAs were carried out by the principal investigator in each hospital, who remained the same throughout the study.
During this phase, we collected prospective data on demographic, clinical and CPR characteristics in infants <32 wGA born in participating centres and requiring neonatal resuscitation included in the study after the parents provided signed informed consent. We excluded infants <32 wGA with biological signs indicative of intrauterine foetal death, with life-limiting anomalies in which neonatal resuscitation is not indicated or whose parents refused participation. We also documented any problems during stabilization, classifying them into 2 possible categories: technical problems (problems due to difficulties using equipment or supplies or limited availability of these resources) or procedure-related (error or omission by the resuscitation team in any of the steps of resuscitation).
The care of infants <32 wGA during resuscitation was delivered by the usual team in each centre, typically comprising 3–4 resuscitation providers (neonatologists, paediatrics residents, neonatal nurses, midwives) trained in neonatal resuscitation and preterm newborn stabilization. During the follow-up, all participating units delivered CPR following the current national and international guidelines at the time of the given phase of the study.9,17 The collection of data during resuscitation was performed by an external observer (usually a neonatologist or nurse with experience in neonatal CPR) using a previously developed form (supplemental material, Appendix B).
The primary objective of the study was to assess 3 particular outcomes: improving axillary temperature at admission of the infant to the NICU, increasing the frequency of reliable pulse oximetry measurement in the first 3 min post birth, and reducing the need of intubation and surfactant administration in the delivery room. These are quality indicators specifically used in neonatal resuscitation and, with some variation, have been analysed in similar studies.18–20 In particular, measurement of axillary temperature at admission is considered a quality indicator given its objectivity and feasibility and the known association between hypothermia and neonatal morbidity and mortality.7,21 On the other hand, measurement of oxygen saturation at 3 min post birth is key to guide decision-making in the management of oxygen therapy in these infants.9 Lastly, avoidance of intubation in preterm infants in the delivery room is a goal that seeks to promote the use of non-invasive ventilation (continuous positive airway pressure [CPAP]) and subsequent administration, if necessary, of surfactant with less invasive methods likely to have fewer or milder side effects.22 The presence of technical or performance problems during resuscitation, improvement in other clinical outcomes during neonatal stabilization and the feasibility of the implementation of these quality improvement tools were established as secondary objectives of the study.
The initial phase was followed by an 8-month intervention period (October 2019–May 2020) with implementation of the systematic use of structured procedure checklists and meetings of the resuscitation team (for checklists and briefings) prior to the stabilization of infants <32 wGA, to systematically check the equipment, supplies and medication, assign roles and go over the sequence before initiating resuscitation. Brief meetings were also held post resuscitation (debriefings) for the team to review its performance and identify strengths and possible areas of improvement (supplemental material, Appendix B). These debriefings were led by the CPR team leader. Before the intervention period, none of the participating units used structured checklist forms or held systematic meetings before or after neonatal resuscitation.
Given the lack of previous published data on the impact of the implementation of a bundle of quality improvement measures on neonatal CPR outcomes, we were not able to estimate the necessary sample size based on formal hypothesis testing results. This is why we opted to design a pre- and post-intervention study in a convenience sample with an equal duration of the pre- and post-intervention phases to prevent seasonal biases. Thus, in the post-intervention period, data were once again collected over 1 year (June 2020–May 2021) on RTRSAs performed in neonatal resuscitation areas and clinical and resuscitation variables in preterm infants with the same methodology applied earlier.
We performed a descriptive statistical analysis and univariate analysis (independent samples tests) to compare study variables pre- and post-intervention. In the case of categorical variables, we used the χ2 test or Fisher exact test, and in the case of numerical variables, the Mann–Whitney U test, Kruskal–Wallis test or Student t test based on whether the data met the corresponding assumptions, considering P values of less than 0.05 statistically significant. The statistical analysis was performed with the Stata software package, version 15.1.
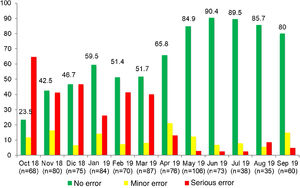
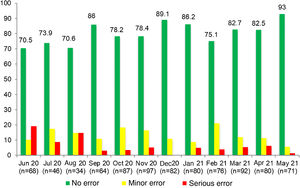
ResultsIn the preintervention phase, a total of 852 RTRSAs of resuscitation areas were performed, of which 534 (62.7%) did not identify errors. We found statistically significant differences (P < .001) compared to the post-intervention phase, during which 877 audits were performed, and 711 found no errors (81.1%) (Table 1).
Comparison of real-time random safety audits of resuscitation areas in pre- and post-intervention phases.
| Overall result | Overall result | P | |
|---|---|---|---|
| Preintervention phase | Post-intervention phase | ||
| N = 852 | N = 877 | ||
| N (%) | N (%) | ||
| No errors | 534 (62.7%) | 711 (81.1%) | <.001 |
| Minor error | 98 (11.5%) | 115 (13.1%) | |
| Serious error | 220 (25.8%) | 51 (5.8%) |
As regards clinical and demographic variables and the maternal-foetal medical history in the included newborns, we did not find significant differences between the pre- and post-intervention phases (Table 2). Seventy nine infants <32 wGA were born in the preintervention phase, and we obtained data for 75 (in 3 cases the parents refused to participate and in 1 case resuscitation data were not collected). In the post-intervention phase, data were collected for 48 of the 51 infants born preterm. In this period, parents refused participation in 2 cases, and resuscitation data were not collected in 1. During the post-intervention phase, the quality improvement measures were implemented in a large percentage of resuscitations with the combined use of briefings, debriefings and checklists in 36 resuscitations (75% of the total) (Table 3).
Comparison of demographic, prenatal and perinatal variables in newborns delivered before 32 weeks’ gestation included in the pre- and post-intervention phases.
| Variable | Results | Results | P |
|---|---|---|---|
| Preintervention phase | Post-intervention phase | ||
| N = 75 | N = 48 | ||
| n (%) | n (%) | ||
| Sex | Male: 39 (52%) | Male: 27 (56.3%) | .645 |
| Ethnicity | Caucasian: 54 (72%) | Caucasian: 29 (60.4%) | .088 |
| Hispanic: 13 (17.3%) | Hispanic: 8 (16.7%) | ||
| African: 6 (8%) | African: 3 (6.3%) | ||
| Maghrebi: 1 (1.3%) | Maghrebi: 6 (12.5%) | ||
| Romani: 1 (1.3%) | Romani: 2 (4.1%) | ||
| Type of pregnancy | Singleton: 56 (74.7%) | Singleton: 33 (68.7%) | .474 |
| Type of delivery | Caesarean: 45 (60%) | Caesarean: 32 (66.7%) | .684 |
| Vaginal: 29 (38.7%) | Vaginal: 15 (31.3%) | ||
| Assisted: 1 (1.3%) | Assisted: 1 (2%) | ||
| Antenatal steroids | Full course: 62 (82.7%) | Full course: 34 (70.8%) | .237 |
| Partial course: 11 (14.7%) | Partial course: 13 (27.1%) | ||
| No: 2 (2.6%) | No: 1 (2.1%) | ||
| Antenatal magnesium sulphate | Yes: 63 (84%) | Yes: 36 (75%) | .219 |
| Gestational age (weeks) | Median: 30 + 5 | Median: 30 + 4 | .208 |
| IQR: 29+6–31+3 | IQR: 29+2–31 | ||
| Birthweight (g) | Mean: 1423.3 | Mean: 1375.6 | .357 |
| SD: 288.8 | SD: 262.9 | ||
| Birthweight percentile | Mean: 47 | Mean: 47 | .975 |
| SD: 23.1 | SD: 24.3 | ||
| Known maternal or foetal disease? | Yes: 51 (68%) | Yes: 36 (75%) | .405 |
| Type of maternal/foetal disease | |||
| Prolonged rupture of membranes | 9 (12%) | 5 (10.4%) | .780 |
| Chorioamnionitis | 11 (14.7%) | 8 (16.7%) | .765 |
| Preeclampsia | 14 (18.7%) | 10 (20.8%) | .767 |
| Umbilical cord or placental disease | 6 (8%) | 4 (8.3%) | .947 |
| Intrauterine growth restriction | 7 (9.3%) | 4 (8.3%) | .850 |
| Other gestational maternal disease | Yes: 5 (6.7%) | Yes: 7 (14.6%) | .149 |
| Cholestasis: 3; hypertransaminasaemia: 1; untreated hypothyroidism: 1 | COVID-19: 4 Bone marrow aplasia:1 Urosepsis:1 Syphilis: 1 | ||
| Other pregestational maternal disease | Yes: 3 (4%) | Yes: 0 | .161 |
| Substance use: 2; uterine malformation: 1 | |||
| Other foetal disease | Yes: 1 (1.3%) | Yes: 0 | .142 |
| Oligoamnios |
IQR, interquartile range; SD, standard deviation.
Use of health care quality tools in resuscitations performed in newborns delivered before 32 weeks’ gestation in the post-intervention phase.
| Tools used | Resuscitations in post-intervention phase |
|---|---|
| N = 48 | |
| n (%) | |
| None | 5 (10.4%) |
| Only briefings | 3 (6.2%) |
| Briefings + checklists | 4 (8.3%) |
| Briefings + debriefings + checklists | 36 (75%) |
We also found similar results in the variables documented during CPR in the pre- and post-intervention phases. Specifically, there were no statistically significant differences in the 3 primary outcomes of the study. The only variables in which we found differences were delay (in seconds post birth) before a reliable pulse oximetry measurement had been achieved if it had not been taken at 3 min post birth, the need of adrenaline during resuscitation and the delay (in minutes post birth) in providing initial information to the family (Table 4).
Comparison of resuscitation variables in pre- and post-intervention phases.
| Variable RCP | Preintervention | Post-intervention | P |
|---|---|---|---|
| N = 75 | N = 48 | ||
| n (%) | n (%) | ||
| Prenatal information | 56 (74.7%) | 40 (83.3%) | .257 |
| Adequate thermal support (exothermic mattress + polyethylene bag in first minute post birth) | 68 (90.7%) | 46 (95.8%) | .283 |
| FiO2 21%–30% at CPR initiation | 75 (100%) | 47 (97.9%) | .209 |
| SatO2 at 3 min post birth | Unknown: 16 (21.3%) | Unknown: 10 (20.8%) | .468 |
| <60: 13 (17.3%) | <60: 14 (29.1%) | ||
| 60−80: 35 (46.7%) | 60−80: 18 (37.5%) | ||
| >80: 11 (14.7%) | >80: 6 (12.5%) | ||
| Delay in SatO2 measurement (if not done at 3 min) (seconds post birth) | Median:300 | Median: 240 | .039 |
| (IQR: 251.5−460) n = 16 | (IQR: 240−300) n = 10 | ||
| Maximum FiO2 | Median: 35 (IQR: 30−40) | Median: 40 (IQR: 30−50) | .093 |
| Time of maximum FiO2 (seconds post birth) | Median: 180 | Median: 225 | .493 |
| (IQR: 1−300) | (IQR: 125−300) | ||
| Highest level of respiratory support | None: 6 (8%) | None: 4 (8.3%) | .612 |
| CPAP: 27 (36%) | CPAP: 14 (29.1%) | ||
| IPP: 34 (45.3%) | IPP: 21 (43.7%) | ||
| Intubation: 8 (10.7%) | Intubation: 9 (18.75%) | ||
| Need of surfactant | 2 (2.7%) | 2 (4.2%) | .647 |
| Chest compressions | 2 (2.7%) | 3 (6.3%) | .326 |
| Need of medication | 0 (0%) | 4 (8.4%) | .011 |
| Respiratory support at admission | None: 7 (9.3%) | None: 5 (10.4%) | .579 |
| CPAP: 58 (77.3%). | CPAP: 34 (70.8%) | ||
| Intubation: 7 (9.3%) | Intubation: 8 (16.6%) | ||
| Supplemental O2: 2 (2.7%) | IPP (w/o intub.): 1 (2%) | ||
| IPP (w/o intub.): 1 (1.3%) | |||
| SatO2 at 5 min post birth | Unknown: 8 (10.7%) | Unknown: 6 (12.5%) | .701 |
| <75: 14 (18.7%) | <75: 12 (25%) | ||
| 75−85: 20 (26.6%) | 75−85: 9 (18.8%) | ||
| >85: 33 (44%) | >85: 21 (43.7%) | ||
| Time of admission (min post birth) | Median: 17 | Median: 20 | .089 |
| (IQR: 10−30) | (IQR: 14−30) | ||
| Temperature at admission (°C) | Median: 36.5 (IQR: 36.3−37) | Median: 36.4 (IQR: 36−36.8) | .799 |
| <32: 0 | <32:0 | ||
| 32−35.9: 8 (10.7%) | 32−35.9: 11(22.9%) | ||
| 36−36.4: 25 (33.3%) | 36−36.4: 14 (29.1%) | ||
| 36.5−37.5: 41 (54.7%) | 36.5−37.5: 22 (45.8%) | ||
| >37.5: 1 (1.3%) | >37.5: 1 (2%) | ||
| Delay in informing family (min post birth) | Median: 17 | Median: 30 | .002 |
| (IQR: 10−30) | (IQR: 12.5−45) | ||
| 1-min Apgar score | Median: 7 (IQR: 6−8) | Median: 7 (IQR: 5−8) | .223 |
| 5-min Apgar score | Median: 9 (IQR: 8−9) | Median: 8 (IQR: 7−9) | .073 |
| CRIB score (12 h post birth) | Median: 0 (IQR: 0−1) | Median: 1 (IQR: 0−2) | .146 |
CPR, cardiopulmonary resuscitation; CRIB, Clinical Risk Index for Babies; FiO2, fraction of inspired oxygen; intub., intubation; IPP, intermittent positive pressure breathing; IQR, interquartile range; min, minutes; SatO2, oxygen saturation; w/o, without.
Statistically significant results are presented in boldface.
When we compared the problems that had emerged during stabilization, we also found no differences between the 2 phases, although there was a higher frequency of technical problems in the preintervention phase compared to the post-intervention phase (14.7% vs 6.2%; P = .151). The incidence of problems in performing the procedure was similar in both phases (10.7% vs 12.5%; P = .754), and the most frequent problem was not achieving intubation in the first attempt (Table 5).
Comparison of problems during resuscitation in the pre- and post-intervention phases.
| CPR variable | Preintervention | Post-intervention | P |
|---|---|---|---|
| N = 75 | N = 48 | ||
| n (%) | n (%) | ||
| Problems during CPR | 18 (24%) | 9 (18.7%) | .492 |
| Technical problem | 11 (14.7%) | 3 (6.2%) | .151 |
| Type of technical problem | Pulse oximeter: 6 | Area not prepared: 1 | |
| Area not prepared: 1 | Temperature: 1 | ||
| Temperature: 1 | Pulse oximeter: 1 | ||
| Gases: 1 | |||
| Tubing: 1 | |||
| Insufficient CPAP pressure: 1 | |||
| Problem with procedure | 8 (10.7%) | 6 (12.5%) | .754 |
| Type of problem with procedure | Pulse oximetry: 4 | >1 intubation attempt: 3 | |
| >1 intubation attempt: 3 | Catheter securement: 1 | ||
| Accidental extubation: 1 | Accidental extubation: 1 | ||
| No polyethylene bag: 1 | Inadvertent FiO2 100%: 1 | ||
| (in 1 CPR, there were 2 errors) |
CPR, cardiopulmonary resuscitation; FiO2, fraction of inspired oxygen.
At present, there is limited evidence suggestive of a short-term improvement in the performance of neonatal CPR with the use of briefings and debriefings, and no evidence on their impact on patient outcomes.7,9 In our study, we were able to demonstrate that their use, combined with the use of checklists and RTRSAs of stabilization areas, was feasible in several level III units, but did not have a significant impact in the main outcomes documented during resuscitation and the hospital stay of newborns <32 wGA. In contrast, Sauer et al. found an improvement in the percentage of newborns with a normal axillary temperature at admission with the implementation of several tools similar to the ones used in our study.18 However, their study included newborns of any gestational age, which may explain the relatively high proportion of normothermia compared to our sample. Other studies that have specifically assessed the usefulness of checklists and debriefings in the resuscitation of preterm infants have also evinced an increase in temperature at admission in these patients, although in some cases the tools had to be implemented for 5 years before significant improvements could be detected.20 This is an aspect that should be taken into account: it takes time for any quality improvement measure to be learned and implemented satisfactorily, and it is possible that, despite the long follow-up in our study, we were still in the initial stages of the learning curve in the application of these tools.
On the other hand, we did not find differences in the delay in the assessment of oxygenation between the newborns included in each phase. However, in the post-intervention phase, we did find a statistically significant difference in the delay in obtaining an adequate pulse oximetry measurement in newborns in whom oxygen saturation had not been measured at 3 months post birth, with a 1-minute reduction compared to the preintervention phase. This difference may be an indirect indicator of improved coordination of care team members and has been described in similar studies.19
Another of the clinical outcomes in which we did not find differences was the need of intubation in this group of infants in the pre- and post-intervention phases of the study. Paradoxically, the percentage of newborns who required intubation and surfactant administration was greater in the post-intervention phase, although the difference was not statistically significant. In any case, both the need of adrenaline (in 4 resuscitations in the post-intervention phase) and the delay in minutes post birth to informing the family (greater in the post-intervention phase) are indirect indicators of a higher complexity in the procedure that could justify these results. Other potential trends that would explain them (albeit not statistically significant) were a lower 5-minute Apgar score and greater delay in the admission to the NICU in the post-intervention phase. Furthermore, despite the apparent homogeneity of the preterm infants included in both phases, the use of a complete course of antenatal steroids was less frequent in the post-intervention phase, a factor that was not statistically significant but that has sufficient clinical impact to be associated with greater respiratory immaturity in the infants included in this phase of the study, and therefore, the increased need of intubation in the delivery room.
Technical or procedural problems emerged in a relatively high percentage of the resuscitations analysed in each phase: 24% in the preintervention phase and 18.7% in the post-intervention phase. It is important to consider that this type of problems, usually due to poor preparation of the resuscitation area, could be solved with the use of a structured checklist and the performance of RTRSAs in these areas. The fact that the RTRSAs of the post-intervention phase evinced an improved preparation of these areas and that structured checklists had been applied before the procedure in a high percentage of resuscitations suggest that both of these measures played a role in the decreased frequency of technical problems encountered in this phase of the study and that the differences were not statistically significant due to the small sample size. Therefore, although we can only discuss this in terms of trends, we believe that the impact in clinical practice on the frequency of technical problems is relevant enough to be taken into account.
In our study, we introduced RTRSAs of resuscitation areas as one of the quality improvement measures to apply in the delivery room, which was a novel approach. In the preintervention phase, only 62.7% of resuscitation areas were completely stocked and correctly prepared to manage a critical situation. In this period, most of the identified minor errors had to do with the preparation of the resuscitation area (incorrect ventilation, oxygenation or suction settings), while serious errors involved the absence of specific equipment, supplies or medication required for resuscitation.23 Other studies in neonates in which RTRSAs were conducted in different areas or procedures in the NICU setting also found surprisingly low percentages of satisfactory audits, consistent with our own findings.12–14 In contrast to these results, in the post-intervention phase the percentage of audits that found no errors increased considerably: 80.1% found no errors of any kind, and there was also an increasing trend in the percentage of RTRSAs without errors throughout this period. Thus, these audits help identify problems in procedures and resources that, once addressed, do not persist in time, as can be seen in Figs. 1 and 2, which present a graphic representation of the results of real-time resuscitation area audits throughout the follow-up.
Based on the large percentage of resuscitations in which all the tools under study were applied in the post-intervention phase, it is reasonable to conclude that their use is generally well accepted by care teams in which they are implemented, as described in the previous literature.24,25 As occurred in past studies, we found lesser compliance with debriefings.24 This could be explained by several known barriers26,27: lack of time, lack of qualified staff to moderate the discussions, lack of an adequate space to conduct debriefings or fear of potential legal ramifications. Notwithstanding, debriefings are essential and some of these barriers should be removed, as several studies have shown that effective debriefings can improve skills at the individual and team levels by up to 25% after their performance.28
LimitationsDespite its multicentre design, the findings of our study may not be generalizable to neonatal units with a different level of care, especially those that manage even more vulnerable infants delivered at lower gestational ages and requiring different care procedures. However, in 2020, the last year for which data are available, of the total children born in the Community of Madrid, 64% were delivered in hospitals offering the same or a lower level of care than the units that participated in this study.29 We believe that our findings could be extrapolated to clinical practice in these centres, as the protocols for the preparation and checking of stabilization areas in the delivery room and the human and material resources of these areas are similar to those reflected in our study. On the other hand, not including instances of CPR in infants born before 28 weeks’ gestation, managed in higher-level care units and more likely to experience problems during stabilization may have played a role in the improvement in the preparation of resuscitation areas and the widespread use of the remaining tools not having the expected impact on clinical outcomes in the newborns included in the study.
Another aspect to consider is the decreased number of infants born preterm in the second phase of the study (a 36% decrease), which was generalised and observed in all participating centres, and likely reflected a decrease in natality associated with the COVID-19 pandemic in Spain from March 2020. This decrease resulted in a difference in the number of neonates included in each of the 2 phases of the study, which makes it difficult to draw rigorous conclusions in their comparison.
We ought to highlight that we did not specifically compare resuscitation procedures in the preintervention phase and procedures carried out in the post-intervention phase during which the quality improvement measures were all applied. Although it not likely due to the difference in the number of resuscitations included in the second phase, this difference could have affected the statistical significance of the different clinical outcomes under study, and may have given rise to selection bias.
As regards the improvement in the percentage of RTRSAs that found no errors in the pre- and post-intervention phases, we ought to highlight that while the delivery room staff was not aware that the study was being conducted, the fact that in some instances the researchers in each centre identified errors and corrected them to prevent the occurrence or persistence of serious unsafe care-related adverse events may have also been a source of bias in the interpretation of these results.
Last of all, another limitation of our study was that we did not obtain video recordings of the instances of resuscitation included in the analysis. Instead, a member of the care team was specifically appointed to record the times at which the different steps of neonatal stabilization were performed. This could have given rise to bias in the documentation of these data, as this method is not as objective or precise as a video recording, which allows in-depth examination of the procedure and more reliable data collection, all the more so in an intervention as complex as neonatal CPR, regarding which several authors have highlighted the difficulty of collecting data and documenting the process accurately.30,31
ConclusionThe implementation of a bundle of health care quality measures in the stabilization of newborns <32 wGA at birth, consisting of RTRSAs of neonatal resuscitation areas and the use of structured checklists, briefings and debriefings, was easy in level IIIA units, although it had no clear impact on the performance of CPR, the measurement of temperature at admission or the respiratory management of these infants during stabilization. The use of RTRSAs in resuscitation areas in the delivery room achieved a significant improvement in their preparation. Further research is required to determine the clinical impact of the application of these tools in the resuscitation of the most vulnerable newborns in the short and the long term.
Conflicts of interestThe authors have no conflicts of interest to declare.