The Newborn Infant Parasympathetic Evaluation (NIPE) index is an instrument that enables continuous, fast and objective assessment of neonatal discomfort. The aim of the study was to analyse changes in NIPE values after performance of blood draws and the factors involved in this variation.
Material and methodsWe conducted a prospective observational study. We included infants admitted to the neonatal intensive care unit between June and December 2021 who underwent blood draws. We recorded demographic data, aspects related to the procedure, the NIPE index and the heart rate at baseline and 1, 2, 3, 4, 5, 10 and 15 min after the procedure.
ResultsThe study included 86 records for 49 patients. In the first 4 min after the procedure, there was a significant decrease in the NIPE index, with a maximum decrease of 22.8% relative to baseline and the nadir at 2.79 min. The decrease in NIPE values was greater in infants born preterm, male, with lower 5-min Apgar scores and following procedures that had been performed previously, after caesarean section or in the morning. There were no differences when the blood draw was obtained during kangaroo care. The correlation between the NIPE index and the heart rate was weak.
ConclusionsAfter a painful procedure, such as a blood draw, the NIPE monitor showed a significant decrease in the first 4 min, which was more pronounced in preterm infants, in repeated procedures or after caesarean delivery. The NIPE index could help identify infants experiencing acute procedural pain, complementing clinical rating scales.
El monitor NIPE (Newborn Infant Parasympathetic Evaluation) es una herramienta rápida, continua y objetiva de evaluación del disconfort neonatal. Los objetivos fueron describir los cambios del NIPE tras una extracción sanguínea y los factores implicados en su variación.
Material y métodosEstudio observacional analítico con recogida de datos prospectiva. Se incluyeron los recién nacidos ingresados en cuidados intensivos entre Junio y Diciembre de 2021a los que se realizó extracción sanguínea. Se recogieron variables demográficas, las relacionadas con la realización del procedimiento, la puntuación NIPE y frecuencia cardiaca previa y en los minutos 1, 2, 3, 4, 5, 10 y 15 posteriores.
ResultadosSe incluyeron 86 registros de 49 pacientes. Durante los primeros 4 minutos tras el procedimiento hubo un descenso significativo en la puntuación NIPE, siendo el descenso máximo de un 22,8% respecto al valor basal, produciéndose el nadir a los 2,79 minutos. El mayor descenso del NIPE ocurrió en pacientes prematuros, varones, con menor Apgar a los 5 minutos, en procedimientos ya realizados previamente, tras cesárea y en horario de mañana. No hubo diferencias con la realización en canguro. La correlación entre NIPE y frecuencia cardíaca fue débil.
ConclusionesTras un procedimiento doloroso, como una extracción sanguínea, el monitor NIPE mostró un descenso significativo los primeros 4 minutos, agudizándose el descenso con la prematuridad, la reiteración de procedimientos o el nacimiento tras cesárea. El monitor NIPE puede ayudar a identificar eficazmente aquellos neonatos que sufren dolor agudo tras un procedimiento, complementándose con las escalas de valoración clínica.
Until the 1980s, the perception of pain in neonates was neglected due to both ignorance and the inability of neonatal patients to convey their pain, a mistake that has since been addressed with the support of the nonmaleficence principle and scientific advances1. Since then, numerous studies have evinced the response of neonates to nociceptive stimuli with autonomic nervous system, hormone and/or behavioural changes from 22 weeks of gestational age, in addition to the short- and long-term benefits of minimising painful stimuli and managing them early if they occur1–3. Accurate assessment of neonatal pain is complex due to the lack of a gold standard that could serve as reference and continues to be one of the most pressing challenges in neonatal care for the purpose of ensuring adequate pain management4–6.
One of the situations in which neonates experience significant pain is during blood collection procedures, which tend to occur repeatedly in patients with prolonged stays, such as preterm or very low birthweight infants7,8. The experience of painful or stressful stimuli in the perinatal period affects neurodevelopment and has a negative impact in the hypothalamic-pituitary-adrenal axis, resulted in abnormal responses to stress in adulthood with an associated increase in cardiovascular risk9,10.
The clinical scales applied for assessment of neonatal pain are used inconsistently, have a subjective, observer-dependent component and are not specific for pain, as their results may be indicative of stress or agitation5,7. A survey of pain assessment practices in Spain found that the CRIES scale was the one used most frequently, and highlighted that two thirds of neonatal units did not carry out pain assessments using scales11. Due to all of the above, it may be worth introducing other, more objective modalities to complement the multimodal scales currently in use to ensure a more thorough assessment.
The Newborn Infant Parasympathetic Evaluation (NIPE) monitor is based on the Analgesia Nociception Index (ANI) developed to assess surgical anaesthesia in adults by analysing changes in heart rate (HR), adapting it to neonatal patients12. The analysis of HR variability allows assessment of the autonomic nervous system activity, which is directly related to the wellbeing of the patient13,14. It yields an absolute value that ranges from 0 to 100, with higher values indicating greater patient comfort. It is an objective measurement available on an ongoing basis and easy to interpret that is not observer-dependent, non-invasive and offers the possibility of assessing parasympathetic activity, both in acute situations with the instantaneous NIPE index and in prolonged ones with the mean NIPE index. The evidence of its application in clinical practice is still scarce, and mainly refers to its use in paediatric intensive care units or during surgical procedures as opposed to the assessment of infants during the stay in the neonatal intensive care unit (NICU)15–17.
The primary objective of our study was to describe changes in the NIPE following blood collection procedures. The secondary objectives were to assess factors associated with changes in the NIPE, including perinatal characteristics, number of blood collection procedures, types of collection procedures, collection of blood during kangaroo care or in the incubator, type of test and timing of the procedure.
Material and methodsSampleThe sample included all neonates admitted to the NICU between June 2021 and December 2021, both included, whose parents signed the informed consent form for participation. The exclusion criteria were: major congenital anomaly, brain injury (grade II-III haemorrhage or white matter lesions), seizures, neuromuscular disease, genetic changes, arrhythmia, administration of antiarrhythmic drugs or drugs acting on the sinus node (including adrenalin, dobutamine and caffeine citrate administered in the past 6 h), recordings interrupted for at least 1 min due to poor signal quality and handling of the infant in the 15 min that followed the collection procedure. No urgent collection procedure was delayed in infants for the purpose of placing a NIPE monitor.
The study was conducted in a level III-A neonatal care unit in Spain that managed 995 deliveries and 103 admissions during the study period.
We calculated the necessary sample size, using the studies of Walas et al.18 and Gendras et al.19 as reference, to detect differences in NIPE values at different time points with a power of 90% with an α of 0.05.
Study designWe conducted a prospective observational and analytical study. The study was approved by the Clinical Research Ethics Committee of our hospital under reference 21-PI082.
Instruments: NIPE monitorThe NIPE monitor reflects the parasympathetic activity of the autonomous nervous system in real time by analysing the HR variability of the previous 64 s, providing one numerical value per second. The electrocardiographic signal is analysed to determine the time elapsed between each of the R waves and spectral analysis of RR series data is used to generate different components, a low-frequency component (0.04−0.15 Hz) mainly related to sympathetic activity, and a high-frequency component (>0.15 Hz) mainly related to parasympathetic activity.
The resulting index ranges from 0 to 100, and higher values correspond to a predominance of parasympathetic activity that represents the wellbeing of the patient. The NIPE monitor presents 2 values: the mean NIPE index, which is the mean of NIPE values in the past 20 min, and the instantaneous NIPE, which is the mean of the values generated in the past 3 min, which is the one indicated to assess acute stress and used in our study.
ProtocolWe used a NIPE monitor (MDoloris Medical Systems, Loos, France) connected to the vital signs monitor (GE Solar 8000i) to obtain absolute instantaneous NIPE values.
We recorded the NIPE index and HR at different time points: at baseline before intervention, immediately before blood collection and at 1, 2, 3, 4, 5, 10 and 15 min after the procedure. The baseline state was defined as the patient being asleep or quietly alert, with no signs suggesting a current experience of stress. We also collected perinatal characteristics for each patient in addition to the method and timing of each collection.
The possible blood collection procedures were: capillary blood for glucose measurement, capillary blood for blood gas/blood chemistry, venous blood for blood chemistry/complete blood count. The nonpharmacological sedation and analgesia protocol of the unit was followed in all collection procedures, with physical containment measures and administration of breast milk or oral sucrose solution 2 min before the procedure. Blood collection was the last procedure performed in any handling of the infant, and no other handling or procedures were performed in the infant for at least the 15 min that followed.
We categorised days as weekday or weekend/holiday and times of day as morning (8:00 am–3:00 pm), evening (3:00 pm–10:00 pm) and night (10:00 pm–8:00 am). The position of the infant during the procedure was in the incubator or in kangaroo care.
We recorded information on the changes in NIPE and HR values as blood was collected. We also compared differences in NIPE values based on perinatal characteristics, type of procedure, repetition of procedures, timing of procedure and infant position (incubator vs kangaroo care). We studied the correlation between the HR variation and NIPE values.
Statistical analysisWe expressed numerical data as mean and 95% confidence interval (CI). We assessed normality with the Kolmogorov-Smirnov test, finding that the NIPE index was the only variable that did not follow a normal distribution, and therefore we analysed this variable using the median and interquartile range and the Wilcoxon test. To assess the correlation between NIPE and HR values, we used the Spearman correlation coefficient. Statistical significance was defined as a P value of less than 0.05.
We performed all the statistical analyses and generated every chart with the software SPSS version 22 (IBM SPSS Statistics).
ResultsAfter applying the inclusion and exclusion criteria, the sample included 86 recordings corresponding to 49 patients.
Table 1 presents the perinatal characteristics of the sample and descriptive data on the performed blood collection procedures.
Clinical characteristics of the sample.
| Gestational age (weeks) | 33 (31−37) |
| Days of life | 3 (1−5) |
| TBirth weight (g) | 2065 (1557−2590) |
| Type of delivery | Uncomplicated: 40.7% |
| Instrumental: 26.8% | |
| Caesarean: 32.5% | |
| 5-min Apgar | 9 (7−10) |
| Infant position | Incubator: 84.8% |
| Kangaroo care: 15.2% | |
| Time of day | Morning: 37.2% |
| Evening: 45% | |
| Night: 17.8% | |
| Day | Weekday: 70.9% |
| Weekend/holiday: 29.1% | |
| Type of collection | Capillary blood gas: 66.2% |
| Capillary glucose: 22.09% | |
| Venepuncture: 11.6% |
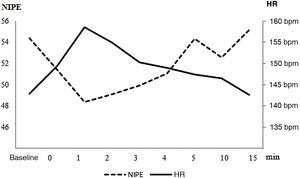
Table 2 and Fig. 1 show the variation in the NIPE and HR values at baseline, at initiation of the procedure and at minutes 1, 2, 3, 4, 5, 10 and 15 after the procedure.
Comparison of mean NIPE and heart rate values at baseline and following blood collection.
| NIPE | HR (bpm) | |
|---|---|---|
| Baseline | 54.37 ± 8.33 | 142.93 ± 16.29 |
| 0 min | 51.45 ± 10.11 | 149.33 ± 16.55 |
| 1 min | 48.39 ± 10.04 | 158.52 ± 19.81 |
| 2 min | 49.10 ± 10.04 | 154.85 ± 19.69 |
| 3 min | 49.92 ± 10.33 | 150.20 ± 20.49 |
| 4 min | 51.06 ± 9.00 | 148.95 ± 19.66 |
| 5 min | 54.35 ± 9.71 | 147.40 ± 17.47 |
| 10 min | 52.55 ± 8.43 | 146.50 ± 16.56 |
| 15 min | 55.18 ± 7.71 | 142.62 ± 18.48 |
bpm, beats per minute; HR, heart rate; min, minutes.
Table 3 presents the NIPE values at the different time points expressed as median and interquartile range, in addition to the results of the statistical analysis of the changes relative to the previous and baseline values.
NIPE values in the different time points (median and interquartile range).
| NIPE | Median (interquartile range) | P (comparison with previous measurement) | P (comparison with baseline) |
|---|---|---|---|
| Baseline | 54.5 (48.0−59.75) | – | – |
| 0 min | 50.5 (44.0−57.75) | <0.01 | <0.01 |
| 1 min | 48.5 (42.25−54.5) | <0.05 | <0.01 |
| 2 min | 49.5 (44.25−57.75) | =0.41 | <0.01 |
| 3 min | 49.0 (43.25−56.75) | =0.83 | <0.02 |
| 4 min | 52.0 (45.5–55.0) | =0.30 | <0.04 |
| 5 min | 54.5 (49.25–60.0) | <0.01 | =0.64 |
| 10 min | 53.0 (46.0–58.75) | =0.07 | =0.22 |
| 15 min | 56.5 (49.5–60.0) | <0.01 | =0.21 |
The third column presents the P value corresponding to the change between the value for that row and the value of the previous row. The fourth column presents the P value corresponding to the change between the NIPE value for the given time point and the baseline NIPE value.
On average, NIPE values decreased a mean of 12.8 points (standard deviation [SD], 7.07) relative to baseline, which amounted to a 22.8 decrease (SD, 14.0%), with the trough value reached at a mean of 2.79 min (SD, 1.19). Table 4 presents the results of the comparison of NIPE values based on perinatal characteristics and timing, infant position and type of procedure (capillary glucose versus all other types of capillary blood collection and venepuncture).
Maximum percent change in the NIPE index after blood collection and minutes elapsed to that trough value based on the clinical variables under study.
| Maximum decrease in NIPE (%) | P | Time to trough NIPE (minutes) | P | ||
|---|---|---|---|---|---|
| Gestational age | ≤32 weeks(n = 32) | 26.67 ± 15.30 | <0.05 | 2.87 ± 1.10 | 0.87 |
| >32 weeks(n = 54) | 20.63 ± 11.97 | 2.84 ± 1.20 | |||
| Birth weight | ≤1500 g(n = 25) | 24.79 ± 13.74 | <0.05 | 2.75 ± 1.23 | 0.65 |
| >1500 g(n = 61) | 18.48 ± 9.61 | 2.88 ± 1.14 | |||
| Sex | Male(n = 50) | 25.38 ± 14.60 | <0.04 | 3.06 ± 1.17 | <0.05 |
| Female(n = 36) | 18.56 ± 8.20 | 2.51 ± 1.12 | |||
| 5-min Apgar | ≤7(n = 28) | 26.85 ± 11.74 | <0.05 | 3.21 ± 1.22 | <0.04 |
| >8(n = 58) | 20.75 ± 14.49 | 2.65 ± 1.08 | |||
| Days of life | ≤2 días(n = 36) | 21.57 ± 13.45 | 0.34 | 2.94 ± 1.39 | 0.44 |
| >2 días(n = 50) | 23.94 ± 14.59 | 2.77 ± 0.91 | |||
| Time of day | Morning(n = 32) | 26.51 ± 13.58 | <0.05 | 3.22 ± 1.24 | <0.05 |
| Evening-night(n = 54) | 20.59 ± 13.41 | 2.69 ± 1.03 | |||
| Day | Weekday(n = 61) | 23.26 ± 14.44 | 0.73 | 2.96 ± 1.12 | <0.04 |
| Weekend/holiday(n = 25) | 22.26 ± 13.93 | 2.36 ± 1.00 | |||
| Collection procedure | Capillary glucose(n = 19) | 13.81 ± 9.40 | <0.01 | 2.14 ± 1.40 | <0.02 |
| All others(n = 67) | 24.80 ± 14.17 | 3.00 ± 1.05 | |||
| Infant position | Incubator(n = 73) | 22.89 ± 14.62 | 0.94 | 2.88 ± 1.14 | 0.37 |
| Kangaroo care(n = 13) | 22.75 ± 10.81 | 2.67 ± 1.30 |
Statistical significance assessed through the P value obtained with the Student t test.
We found significant differences in NIPE values in the first 48 h post birth between infants delivered vaginally (n = 14) versus by caesarean section (n = 22) in the maximum percent decrease (19.84% ± 11.20% vs 25.01% ± 2.76%; P < 0.05) and the minutes elapsed from collection to the NIPE trough (2.67 ± 1.41 vs 3.50 ± 1.14; P < 0.05).
As regards repeated procedures, we found a mean percent decrease in NIPE values after the first blood collection (n = 56) of 19.9% compared to 28.3% after subsequent collections (n = 29), with a mean difference of 8.4 (95% CI, 0.8–17.9; P < 0.05). There were no significant differences in the sample in the time elapsed to the NIPE trough value between the first collection procedure and subsequent procedures (2.86 vs 2.69 min; P = 0.19).
The correlation coefficient for the association between changes in NIPE values and changes in HR values following collection procedures was –0.24 and statistically significant.
DiscussionThe NIPE monitor, recently introduced in clinical practice, allows a rapid and objective evaluation of neonatal comfort. Following procedures that cause acute pain, such a blood collection, there are significant changes in instantaneous NIPE values in the first four minutes, with trough values occurring at 2.79 min. The factors associated with a greater decrease in NIPE values are preterm birth, male sex, caesarean delivery, lower 5-min Apgar scores, capillary blood gas analysis or venepuncture, performance of collection in the morning and performance of repeated procedures. This is the first study in the Spanish language literature and one of the first at the international level to assess NIPE values following performance of a painful procedure in the NICU setting, and we did not find any evidence on the performance of repeated procedures or the type of delivery in relation to changes in NIPE values.
Based on our findings, NIPE monitoring may be an effective tool for detection of neonatal discomfort in the minutes following the most frequent type of painful stimulus in the UCIN setting, blood collection procedures, on which conclusive evidence had not been previously published. On one hand, there are studies that support its efficacy, such as the ones conducted by Walas et al.18 (although the sample size is small and it only assessed the first three minutes after the procedure) and by Gendras et al.19, both of which, however, found a low specificity using neonatal pain scales as reference, such as the Premature Infant Pain Profile (PIPP), the revised version of the PIPP (PIPP-R) or the Face, Legs, Activity, Cry, Consolability (FLACC) scale. While Cremillieux et al.20 did not find differences in the NIPE values obtained at baseline and 3 min after blood collection, nor a correlation with the PIPP-R, leading the authors to conclude that the NIPE was not a reliable tool, in our study the greatest median difference was found in the first 2 min. The trough NIPE value was consistent with the minimums reported by Gendras et al. and Walas et al, and was in between the values found in these two studies. Based on the current evidence, there does not seem to be a reliable correlation between the NIPE index and clinical scale scores. One possible explanation is that behavioural or facial gestures reflect subcortical activity, whereas many painful stimuli are processed at the cortical level with no discernible behavioural changes5; another possibility is that in stressful situations, such as a prolonged stay in the NICU, the amplitude of the cortical response to painful stimuli increases, with no correlation to behaviour21.
When it came to differences based on gestational age, we found a greater decrease in the NIPE index in preterm infants, probably on account of the immaturity of the autonomic nervous system and especially the parasympathetic component, as the myelination of the vagus nerve starts on week 2522,23. In the first days of life there is a predominance of sympathetic activity, especially in preterm infants, who exhibit few high-frequency waves (which arise from parasympathetic activity). Later, as the autonomic nervous system matures, the parasympathetic system lags behind the sympathetic system, and takes more weeks to catch up to the parasympathetic tone in term infants, with significant differences found through at least 46 weeks of postconceptional age24,25. Neonates with lower Apgar scores exhibited greater decreases in NIPE values following blood collection procedures, an aspect that had not been studied in the past and from which few conclusions can be drawn given the limited sample size, as only one of the three patients received a diagnosis of hypoxic-ischaemic encephalopathy (HIE) of moderate severity. In the context of HIE, there is previous evidence that HR variability is a predictor of poor outcomes if there is a predominance of parasympathetic activity26,27.
As for differences based on sex, it is well known that the maturation process is faster in girls compared to boys and that boys are more likely to experience neurodevelopmental abnormalities due to a variety of genetic, hormonal and environmental factors28. Neuroimaging reveals greater development in human males of regions like the amygdala and hippocampus, associated with the integration and memory of emotions and the response to them, while in females there is greater development of cortico-subcortical connections29. At the functional level, there is evidence that female foetuses are more capable of responding and adapt better to stressful situations, with higher serum catecholamine levels, all of it due to an increased activation of the autonomic nervous system in response to a potentially stressful situation30,31.
In our sample, we did not observe differences based on whether the procedure was performed during kangaroo care or with the infant in the incubator, which could be attributed to the small size of the kangaroo care subgroup. Other studies have found benefits with kangaroo care, such as the one conducted by Choudhary et al.32, who found a shorter duration of crying after the heel prick and better recovery time in the kangaroo care group, and the study by Butruille et al.33, which found higher baseline NIPE values in the kangaroo care group in both infants and mothers, reflecting a reduction in maternal anxiety.
The repetition of painful stimuli causes a greater decrease in NIPE values, an outcome that has been studied more extensively in animals, with results evincing a direct association between the number of painful stimuli and neuronal apoptosis, and also demonstrated in humans, with evidence of a reduced volume in the thalamic regions involved in somatosensory processing34,35. Gokulu et al.36 made the closest approximation by assessing it based on clinical manifestations in term neonates, finding higher pain scale scores in infants who received more painful stimuli. In relation to the other variables under study, one salient finding was a lower decrease of NIPE values in the evening and night shifts, possibly associated with the decreased environmental noise and frequency of interventions in the NICU in this timeframe; however, a lower use of sedation and analgesia in night shifts has been previously described37. As regards NIPE values in infants delivered by caesarean section, the most similar previous study used HR variability analysis and also found a greater percentage of parasympathetic activity in infants delivered vaginally38; interestingly, the conclusion is the opposite with the use of clinical scales, possibly on account of what was explained above regarding the neuroanatomical pathways involved in the features contemplated by these scales5,39. Lastly, the observed differences between blood collection procedures are easily explained by the lesser blood volume required for capillary blood glucose measurement, on account of which the lancet used to prick the skin is smaller and the procedure briefer.
One of the limitations of our study is the lack of comparison with the application of clinical scales, and therefore of an assessment of pain severity; however, the studies published to date have not found a correlation with NIPE values. Another limitation is that the sample size was not large, although it was greater compared to the studies of Walas et al. or Cremillieux et al. and similar to the size in the study by Gendras et al.18–20. It is important to consider that stronger nociceptive stimuli than those caused by blood collection would be reflected in greater variation in the instantaneous NIPE, as this measure constitutes the mean value for the last 3 min. Due to the lack of a gold standard for the assessment of neonatal pain, it is not possible to draw firm conclusions on the superiority of one method over another, and the combination of all possible methods is the optimal approach to the assessment of the fifth vital sign: pain.
In conclusion, we would highlight that the instantaneous NIPE is an effective tool in the assessment of procedural pain associated with collection of blood samples in the NICU, showing a significant decrease in the first 4 min following the procedure that is more marked in infants born preterm, male, delivered by caesarean section, with a lower 5-min Apgar score and that have previously undergone another blood collection procedure, as well as in procedures performed in the morning shift.
Conflicts of interestThe authors have no conflicts of interest to declare.
Previous presentation: Partial results from this study were presented at the XXVIII Congress of the Sociedad Española de Neonatología, 2021.