It is known that infants with viral respiratory infections severe enough to require hospital admission have a high risk of developing recurrent wheezing. Few data have been published on unselected populations. The main aim of this study was to analyse symptomatic and asymptomatic respiratory viral infections during the first year of life in a cohort of infants, recruited at birth, and the development of recurrent wheezing.
Patients and methodsA total of 302 newborns were recruited. A nasopharyngeal aspirate was taken when the patients had a respiratory infection, as well as in the visits for vaccination at 2, 4, 6, and 12 months. RT-nested PCR assays were performed to detect 16 viruses.
ResultsA total of 1293 samples were analysed (1005 healthy controls and 288 respiratory infections). Samples taken during routine check-ups were positive in 30.8% of cases, while those with respiratory infection were positive in 77.8%, P<.001 (OR: 3, 95% CI: 2.4–3.8). A total of 239 (79%) infants had at least 1 positive respiratory viral infection detected. The most frequent virus (71%) was rhinovirus (RV). Recurrent wheezing was found in 27 (11%) children during their first year of life (1.2 episodes, SD 2.9). Recurrent wheezing was present in 58.3% of patients admitted to hospital during their first viral infection, vs. 8.6% of infants when the first infection was mild or who had asymptomatic viral detection, P<.001 (OR: 2.18; 95% CI: 1.05–4.5).
ConclusionsIn our series, severe respiratory infections leading to hospitalisation in the first months of life are risk factors for developing wheezing, but not in the case of mild RV infections.
Las infecciones respiratorias virales que requieren hospitalización parecen conferir riesgo de desarrollar sibilancias recurrentes, pero existen pocos datos publicados en poblaciones no seleccionadas por tener factores de riesgo. Nuestro objetivo principal fue analizar si las infecciones respiratorias virales sintomáticas y asintomáticas, de diferente gravedad, durante el primer año de vida en una cohorte de recién nacidos, suponen un mayor riesgo de sibilancias recurrentes.
Pacientes y métodosSe incluyeron 302 recién nacidos. Se recogió aspirado nasofaríngeo a los niños cuando presentaron una infección respiratoria y de forma periódica en los controles de salud (2, 4, 6 y 12 meses). Se estudiaron 16 virus respiratorios mediante reacción en cadena de polimerasa (PCR).
ResultadosSe analizaron 1.293 muestras (1.005 controles de salud y 288 infecciones respiratorias). El 30,8% de las muestras tomadas en los controles de salud fueron positivas, frente a un 77,8% en las infecciones respiratorias, p<0,001 (OR: 3, IC 95%: 2,4-3,8). Un total de 239 (79%) lactantes tuvieron al menos una detección viral positiva durante el primer año de vida. El virus más frecuentemente identificado (71%) fue el rinovirus (RV). En 27 lactantes (11%) se detectaron sibilancias recurrentes durante su primer año de vida (2,9 DE: 1,2 episodios). El 58,3% de los lactantes cuya primera infección respiratoria requirió hospitalización desarrollaron sibilancias de repetición, frente al 8,6% de los niños cuya primera infección fue leve o asintomática, p<0,001 (OR: 2,18; lC 95%: 1,05-4,5).
ConclusionesEn nuestra serie, las infecciones respiratorias virales graves en los primeros meses de vida supusieron un factor de riesgo para desarrollar sibilancias recurrentes. No ocurrió lo mismo con las infecciones respiratorias leves.
Acute respiratory infections (ARIs) are a major reason for health care use in infants and constitute a substantial economic burden.1,2 Although infections in this age group are most frequently of a viral aetiology,3,4 inappropriate antibiotic use continues to be widespread. Techniques based on the polymerase chain reaction (PCR) have made the aetiological diagnosis of ARIs possible in children, and made us aware of the high prevalence of asymptomatic viral infections.5,6 Respiratory syncytial virus (RSV), human metapneumovirus (hMPV) and influenza virus (FLU) have been clearly identified as respiratory pathogens, and more recent studies have been analysing the role of other viruses such rhinovirus (RV) and human bocavirus (hBoV), although the high prevalence of coinfection with other respiratory viruses or their detection in asymptomatic patients pose challenges to the interpretation of these positive results.7,8
It is known that children with severe RSV infection that require hospitalisation are at high risk of developing asthma in the long term, even as late as age 18 years, as Sigurs et al. demonstrated.9 There is also evidence of an increased risk of recurrent wheezing up to age 5 years in infants hospitalised due to bronchiolitis associated to hMPV.10 There are fewer studies in children that have not been admitted to hospital, among which we would like to mention the classic studies by Stein et al., who analysed an unselected cohort and found that milder infections by RSV also increased the risk of developing wheezing.11 In recent years, the role of RV as a long-term predictor of future asthma has also been investigated. A study of particular interest on the subject is that of Lemanske et al.,12 whose cohort of infants that had a RV infection with wheezing in the first year of life were at increased risk of having developed wheezing by age 3 years, with an odds ratio of 10. Most of the studies that assess the risk of developing asthma or wheezing have been conducted in selected high-risk populations.13,14 Furthermore, little is known about mild viral respiratory infections managed in outpatient settings in relation to the future development of wheezing, not to mention asymptomatic viral infections, although it is generally assumed that risk increases with increasing severity. The objective of our prospective study was to analyse asymptomatic and symptomatic infections of varying severity in a cohort of newborns during the first year of life and assess their role in the development of recurrent wheezing. Our secondary objective was to analyse the aetiology and clinical characteristics of these infections.
Patients and methodsRecruitment of newborns and study designWe conducted a prospective cohort study in newborns to analyse the association between past viral infections of varying severity and the development of recurrent wheezing. We requested the participation of all newborns delivered in four health care centres in the Área Sur health zone of Madrid during their first neonatal visit (7–14 days of life). The parents or guardians signed an informed consent form and completed a questionnaire of epidemiological data (history of asthma and atopy in the family, exposure to tobacco smoke, pregnancy and delivery data, number of siblings). Nasopharyngeal aspirate (NPA) specimens were collected during this visit and routine checkups performed at ages 2, 4, 6 and 12 months. Furthermore, if the infants developed respiratory symptoms, they were evaluated by a paediatrician, with collection of an additional NPA sample.
We considered children with specimens collected during routine well-child checkups healthy controls, even if they presented with isolated rhinorrhoea. We defined asymptomatic infection as detection of a virus in the NPA of one of these patients. We defined clinically significant respiratory infection as a case that met the clinical criteria for respiratory infection presented in Table 1. We defined “first detected viral infection” as PCR detecting a respiratory virus in a sample for the first time in the life of a child, whether the infection was asymptomatic, symptomatic and managed at the outpatient level, or symptomatic and requiring hospital admission.
Clinical manifestations of respiratory infectionWhen a child met the criteria for respiratory infection described in Table 1, the paediatrician conducted an evaluation, a clinical data form was completed, and a NPA sample collected. If the infant required admission to hospital, data for the most relevant variables were also collected in another form. Isolated rhinorrhoea was not considered a symptomatic respiratory infection. We differentiated between the following forms of respiratory infection: upper respiratory tract infection, defined as infection meeting the criteria specified in Table 1 in the absence of respiratory distress or wheezing. We defined bronchiolitis as the first episode of respiratory infection that manifested with respiratory distress and wheezing. Subsequent episodes of similar characteristics were classified as recurrent wheezing. Episodes manifesting with inspiratory dyspnoea and wheezing were classified as laryngotracheobronchitis. Laryngitis was defined as inspiratory dyspnoea without wheezing. We defined pneumonia as evidence of focal infiltrates or consolidation in chest radiography in the absence of wheezing.
We classified all infections requiring hospital admission as severe, and the rest as mild.
Sample collectionWhen the requisite criteria were met, a NPA sample was collected and kept at 4°C to await transportation to the Influenza and Respiratory Viruses Laboratory of the Centro Nacional de Microbiología (National Centre of Microbiology). In hospitalised patients, the NPA sample was collected between 7 and 8AM of the morning following admission and kept refrigerated until it was transported.
Virology testingThree RT-nested PCR assays were used to test for 16 respiratory viruses. Reverse transcription (RT) and the first amplification reaction were performed with OneStep RT-PCR kits (Qiagen). Influenza A, B and C were detected by a previously described PCR assay.15 A second multiplex PCR assay was used to detect parainfluenza virus (PIV) 1 through 4, coronavirus 229E and OC43, enterovirus and RV.16 The presence of RSV-A and B, hMPV, hBoV and adenovirus (ADV) was assessed with a third RT-nested PCR assay using the BRQ method.3
Statistical analysisWe have expressed discrete data as percentages and continuous data as mean and standard deviation. We compared clinical and laboratory characteristics using the Student's t, Mann–Whitney U, chi-square and Fisher exact tests. We defined statistical significance as a P-value of less than .05 in any of the tests. The statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) version 13.0.
ResultsEpidemiological characteristics of the cohortWe recruited a total of 302 infants at birth, of which 55.3% were male (167), 80% born to Spanish parents, and 14% of Latin-American ancestry. Thirty-five percent of infants were exposed to tobacco smoke; 36% of mothers and 37% of fathers had asthma and/or atopy. Only 2 patients were born preterm at a gestational age of 33 weeks. All infants but 7 completed the yearlong followup. Those 7 patients did not attend the 12-month checkup, but we contacted their paediatricians to obtain information about their clinical outcomes, so they were ultimately included in the analysis.
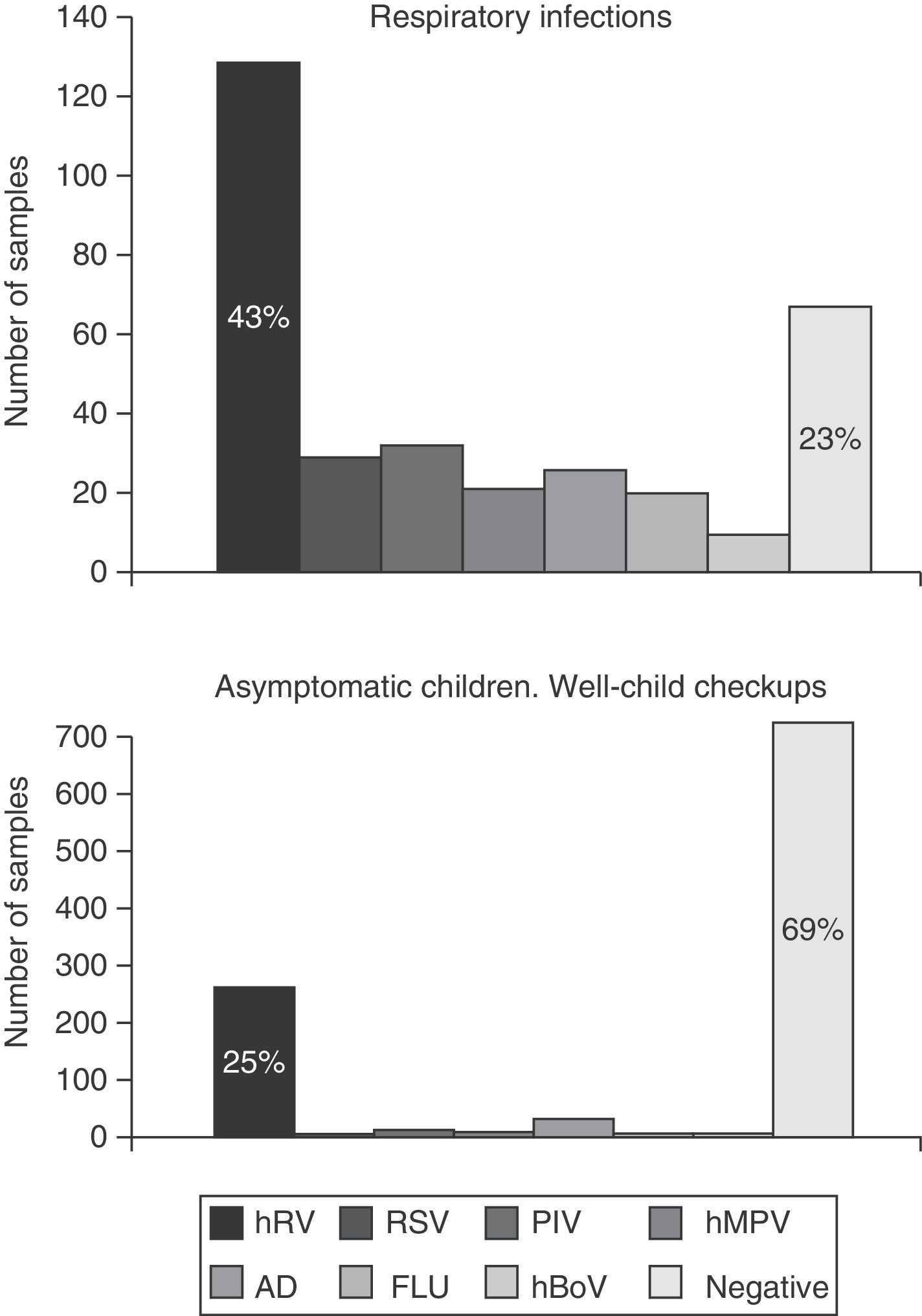
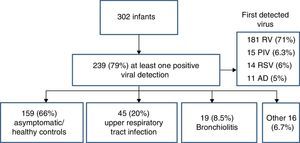
Virology specimens and resultsA total of 1,239 samples were collected, 1,005 during routine visits and 288 when a respiratory infection was suspected. Of the samples collected during the well-child checkups, 30.8% (310) tested positive, while viruses were detected in 77.8% of respiratory infection cases (224/288), P<.001 (odds ratio, 3; 95% confidence interval, 2.4–3.8). The frequency of viral detection in asymptomatic children (healthy controls) increased proportionally with age from 20.8% in newborns aged 15 days to up to 42% in infants aged 12 months. The virus identified most frequently was RV, found in 82% (254) of cases. Other viruses were found in a small proportion of cases: ADV (9.7%), PIV (4.2%), hBoV (3.2%), hMPV (2.9%), FLU (2.2%) and RSV (1.6%) (Fig. 1).
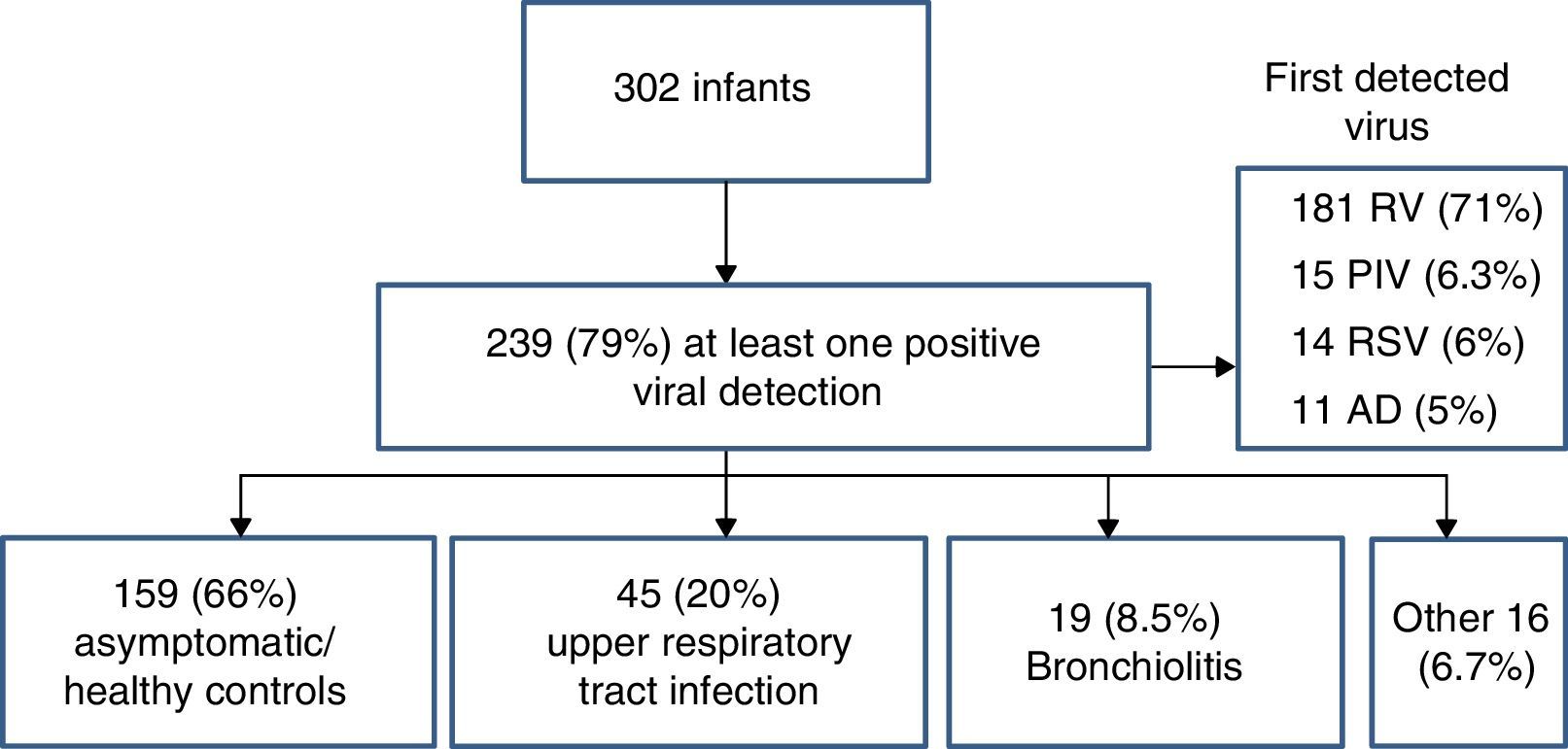
First detected viral infection and patient outcomesOf the 302 infants included in the study, 239 (79%) had at least one positive viral detection in the first year of life (Fig. 2). The virus detected most frequently was RV (181 samples, 71%), followed by PIV (6.3%), ADV (5%) and RSV (6%). Coinfections were only detected in 21 cases (8.8%). The mean age at detection of the first viral infection was 3.4 months (SD, 2.8). The first detected viral infection was associated with upper respiratory tract infections in 45 patients (20%), bronchiolitis in 19 (8.5%) while remaining 159 samples (70%) corresponded to asymptomatic patients and had been collected during routine well-child checkups.
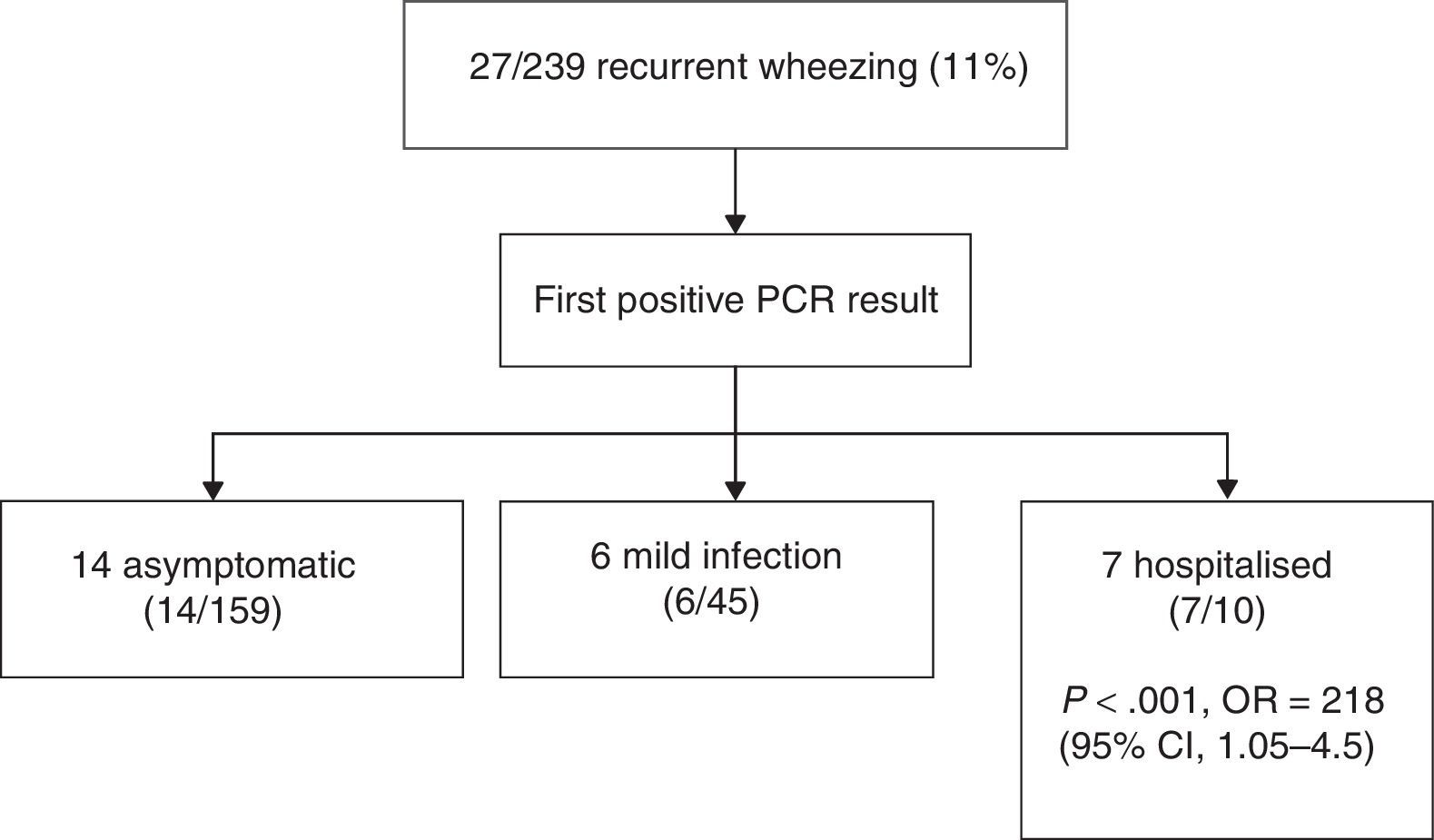
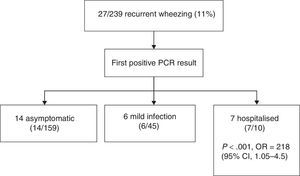
We analysed the outcomes of infants that had at least one positive specimen, and found that 27 (11%) developed recurrent wheezing in the first year of life (mean±SD, 2.9±1.2 episodes; range, 2–5) (Fig. 3). Rhinovirus was the first virus detected in 18 patients (66%), of who 14 (52%) were asymptomatic at the time of the specimen collection (routine checkup). The mean age of infants that developed recurrent wheezing with their first positive viral detection was 2 months (SD, 1.3; all but three were at least 3 months old). Ten of the 27 infants with episodes of bronchiolitis required admission (37%). The most frequently involved virus was RV, both in mild bronchiolitis cases managed at the outpatient level (11/17, 69%) and in severe cases that required hospitalisation (7/10, 70%).
Recurrent wheezing episodes (at least one following the initial bronchiolitis) occurred in 58.3% of infants in who the first viral detection corresponded to a severe infection that required admission, compared to 8.6% of infants whose first detection corresponded to a mild infection managed at the outpatient level or an asymptomatic infection (P<.001; odds ratio, 2.18; 95% CI, 1.05–4.5). A total of 5 infants received montelukast or budesonide during the followup (18%, 3 admitted to hospital and 2 managed at outpatient level at first positive viral detection). There were no differences in the family history of asthma or atopy between children with and without recurrent wheezing.
Characteristics of infections managed in outpatient and inpatient settingsThere were 288 episodes of infection managed in outpatient settings that corresponded to 160 infants (53% of the total sample) who had between 1 and 6 episodes each (mean±SD, 1.6±1). The most frequent symptoms were: fever in 90 cases (38%), rhinorrhoea in 224 (96%), cough in 223 (96%), respiratory distress in 51 (21%) and nonspecific symptoms in 35 (15%). The most frequent diagnoses were upper respiratory tract infection (147, 51%) and bronchiolitis (48, 16%), with a lesser incidence of recurrent wheezing, laryngitis and pneumonia. Only 2 patients had infiltrates on radiologic examination, and both required hospital admission during the followup. The viruses detected in these infections were RV in 127 cases (55.8%), followed by PIV (13%), RSV (12.5%), hMPV (8.9%) and FLU (8%) (Fig. 2). The causative agent of upper respiratory tract infection was RV in 61% of cases, followed by PIV (13%) and ADV (11%). When it came to bronchiolitis, RV was detected in 53%, RSV in 25% and hMPV in 8% of cases.
We analysed a total of 19 hospital admissions corresponding to 14 infants (14/302; 4.6% of the cohort; 5.8% of infants with a positive viral detection). Five patients were admitted more than twice in the first year of life. Fifty-eight percent of admitted patients (11) were female. The mean age of admission was 3.6 months (SD, 2.5 months; range, 6 days–8 months). Forty-two percent presented with fever (mean, 38.6°C; SD, 0.9°C) lasting 3.5 days (SD, 2). Sixty-eight percent (13/19 episodes) required oxygen supplementation for a mean of 5 days (SD, 3). Twenty-six percent (5/19) had infiltrates detected by radiology. The diagnoses were bronchiolitis in 12 cases (63%), wheezing episode in 3 (15%), upper respiratory tract infection in 3 and pneumonia in 1. Only 3 infants received antibiotics. Respiratory syncytial virus was detected in 42% of the cases and RV in 52%. Viral coinfection was detected in 4 cases.
DiscussionA considerable number of infants test positive for at least one respiratory virus in the first year of life, and we observed a percentage of 79% in our cohort. More than half of the cohort experienced at least one symptomatic infection treated at the outpatient level, with a mean of 1.6 symptomatic infections a year (range, 1–6), while approximately 5% required hospital admission. Rhinovirus was the virus detected most frequently in symptomatic (43%) as well as asymptomatic (25%) infants.
Eleven percent of the infants with positive virology results went on to develop recurrent wheezing in the first year of life, but the risk was much higher in patients that required admission due to bronchiolitis (58%; odds ratio, 2.18) compared to patients whose first infection was mild or asymptomatic.
Our data are consistent with the findings of the study conducted by Rhedin et al., who analysed a group of paediatric outpatients with respiratory infections and a group of asymptomatic controls. Rhedin et al. detected respiratory viruses in 72.3% of cases (n=151) and 35.4% of controls (n=74) (P=.001). Rhinovirus was the virus identified most frequently in both cases and controls (47.9 and 21.5%, respectively).6 Although infections by RV are common, a recent study that used nucleotide sequencing demonstrated that the prolonged presence of rhinovirus RNA in the respiratory tract following an upper respiratory tract infection is rare in healthy infants (<5%). In these infants, the detection of RV RNA in repeat samples at least 30 days apart most likely corresponded to new infections.17 Another study found that the detection of the same virus strain more than 2 weeks apart was unusual.18 In our study, we found that RV was detected in a significant proportion of infants that were classified as healthy (routine vaccination visit), probably because we did not take isolated rhinorrhoea into account, and this is a frequent symptom in children that does not contraindicate vaccination. However, it is possible that rhinorrhoea is actually a presenting symptom in mild RV infections. Unfortunately, we have no data on the percentage of infants considered healthy controls that had rhinorrhoea as the sole symptom. This is consistent with the hypothesis of Jartti et al. according to which RV always causes an actual infection, which may or may not be clinically relevant.5 Although there are authors like Jansen et al.,19 who found a higher RV load in children with acute symptomatic infections compared to asymptomatic children, or Utokaparch et al.,20 who detected significantly higher RV loads in infections of the lower respiratory tract, there are others, like Takeyama et al.,21 who have not found a correlation between viral load and disease severity in RV infections. At any rate, the clinical significance of viral loads in respiratory samples is not well established, and we did not measure viral loads in our patients.
We also ought to highlight the small percentage of coinfections found in our cohort (8.8%), as other case series, including previous studies by our research group, found a percentages of coinfection of up to 46% in hospitalised children with rhinovirus infections.22 We believe that the age of our patients (a substantial proportion of the specimens were collected from infants aged less than 6 months) and our decision to include mild and asymptomatic infections with positive virology may account for the low proportion of coinfection.
In a cohort study of 140 newborns with a design similar to our own, Van der Gugten et al.23 found that RV infections manifesting with wheezing in the first year of life were a risk factor for developing recurrent wheezing in the future. Although they did not assess the severity of acute episodes, mild infections without wheezing were not associated with an increased risk of recurrent wheezing. These authors reached the conclusions we have reached based on our cohort, in which infections by RV or RSV that were sufficiently severe to cause bronchiolitis requiring hospital admission were a risk factor for developing wheezing as early as the first year of life. The followup of our cohort in the immediate future will allow us to learn whether the risk of wheezing continues to be higher in early childhood. As Lemanske et al. suggested,12 the severity of infection is a risk factor for the development of asthma and recurrent wheezing. Various studies have demonstrated that severe bronchiolitis associated to RV infection increase the risk of asthma, but as we commented above, these studies were conducted in selected populations.24–26 Our case series, which analysed symptomatic and asymptomatic infections and severity at an early age (less than 3 months in most first episodes) in an unselected population contributes a broader picture on the subject.
Although our study has strengths, such as its unselected sample and testing for all respiratory viruses (as opposed to only the most prevalent) and for the presence of asymptomatic infection, we ought to comment on certain methodological aspects. We did not collect weekly samples to study viral shedding and its duration, and we also did not perform genotyping of RV. The duration of followup was short, and the number of patients with recurrent wheezing small. Nevertheless, we believe that the association found in the study is worth considering, and that we will be able to reach broader conclusions in upcoming years. Until then, we can state that respiratory infections in the first months of life that are severe enough to require hospitalisation are a risk factor for the development of recurrent wheezing.
FundingThis study was partially funded by the Fondo de Investigaciones Sanitarias (Health Research Fund), project PI12/0129; the 2011 Research Grant of the Asociación Española de Pediatría (Spanish Association of Paediatrics [AEP]); and the 2011 Research Grant of the Sociedad Española de Neumología Pediátrica (Spanish Society of Paediatric Pulmonology).
Conflict of interestsThe authors have no conflict of interests to declare
Luis Carlos Aragón: Centro de Salud Leganés Norte, Leganés, Madrid, Spain.
Ana María Berroya: Centro de Salud Leganés Norte, Leganés, Madrid, Spain.
Laura Carretero: entro de Salud Leganés Norte, Leganés, Madrid, Spain.
José María de Cea: Department of Paediatrics, Hospital Severo Ochoa, Leganés, Madrid, Spain.
Sandra García-Estevez: Centro de Salud Leganés Norte, Leganés, Madrid, Spain.
Gema García-Ron: Centro de Salud María Jesús Hereza, Leganés, Madrid, Spain.
Mónica Gonzalez-Esguevillas: Centro de Salud María Jesús Hereza, Leganés, Madrid, Spain.
Carmen Luján: Centro de Salud Jaime Vera, Leganés, Madrid, Spain.
Mar Molinero: Influenza and Respiratory Viruses Laboratory, Centro Nacional de Microbiología, Instituto de Salud Carlos III, Madrid, Spain.
Fermín Vinaixa: Centro de Salud Leganés Norte, Leganés, Madrid, Spain.
Members of the Working Group on Recurrent Wheezing are listed in Appendix A.
Please cite this article as: Calvo C, Aguado I, García-García ML, Ruiz-Chercoles E, Díaz-Martinez E, Albañil RM, et al. Infecciones virales respiratorias en una cohorte de niños durante el primer año de vida y su papel en el desarrollo de sibilancias. An Pediatr (Barc). 2017;87:104–110.