To determine the prevalence and risks factors of vitamin D deficiency, as well as its relationship with morbidity and mortality in a PICU.
Materials and methodsAn observational prospective study in a tertiary children's University Hospital PICU conducted in two phases: I: Cohorts study, and II: prevalence study. The study included 340 critically ill children with ages comprising 6 months to 16 years old. Exclusion criteria: Chronic kidney disease, known parathyroid disorders, and vitamin D supplementation. Total 25-hydroxyvitamin D [25(OH)D] was measured in the first 48h of admission to a PICU. Parathormone, calcium, phosphate, blood gases, blood count, C-reactive protein, and procalcitonin were also analysed. A record was also made of demographic features, characteristics of the episode, and complications during the PICU stay.
ResultsThe overall prevalence rate of vitamin D deficiency was 43.8%, with a mean of 22.28 (95% CI 21.15–23.41)ng/mL. Patients with vitamin D deficiency were older (61 vs 47 months, P=0.039), had parents with a higher level of academic studies (36.5% vs 20%, P=0.016), were admitted more often in winter and spring, had a higher PRISM-III (6.8 vs 5.1, P=0.037), a longer PICU stay (3 vs 2 days, P=0.001), and higher morbidity (61.1% vs 30.4%, P<0.001) than the patients with sufficient levels of 25(OH)D. Patients who died had lower levels of 25(OH)D (14±8.81ng/mL vs 22.53±10.53ng/mL, P=0.012). Adjusted OR for morbidity was 5.44 (95% CI; 2.5–11.6).
ConclusionsVitamin D deficiency is frequent in critically ill children, and it is related to both morbidity and mortality, although it remains unclear whether it is a causal relationship or it is simply a marker of severity in different clinical situations.
Determinar la prevalencia y factores de riesgo del déficit de vitamina D (VDD) en una unidad de cuidados intensivos pediátricos (UCIP), así como su relación con la morbimortalidad durante el ingreso.
Material y métodosEstudio observacional prospectivo realizado en la UCIP de un hospital terciario en 2 fases: i: estudio de cohortes, y ii: estudio de prevalencia. Se incluyó a 340 niños > 6 meses, excluyendo a aquellos con enfermedad renal crónica, trastornos paratiroideos y suplementación con vitamina D. Se realizó medición de 25-hidroxivitamina D total (25[OH]D) en las primeras 48 h del ingreso, parathormona (PTH), calcio, fósforo, gasometría venosa, hemograma, proteína C reactiva y procalcitonina. Se registraron datos sociodemográficos, características del episodio y complicaciones.
ResultadosLa prevalencia de VDD (<20ng/ml) fue del 43,8%, con media de 22,28 (IC del 95%, 21,15–23,41) ng/ml. Los pacientes con déficit fueron de mayor edad (61 vs. 47 meses, p=0,039), sus padres tenían un mayor nivel académico (36,5% vs. 20%, p=0,016), ingresaron más frecuentemente en invierno y primavera, obtuvieron mayor puntuación PRISM-III (6,8 vs. 5,1, p=0,037), mayor estancia (3 vs. 2 días, p=0,001) y morbilidad (61,1% vs. 30,4%, p<0,001) que los pacientes con niveles suficientes (≥ 20ng/ml). Los pacientes fallecidos tuvieron niveles inferiores de 25(OH)D (14±8,81ng/ml vs. 22,53±10,53ng/ml, p=0,012). La OR ajustada para la morbilidad fue 5,44 (IC del 95%, 2,5-11,6).
ConclusionesEl VDD es frecuente en pacientes críticos pediátricos y está relacionado con la morbimortalidad en UCIP, aunque queda por esclarecer si se trata de una relación causal o es simplemente un marcador de gravedad en diferentes situaciones clínicas.
Vitamin D deficiency (VDD) is the most frequent vitamin deficiency in the general population. Its prevalence ranges between 25% and 35% depending on latitude, age, race, sociocultural habits and nutrition.1
Vitamin D (VD) has a variety of effects in the body. In addition to its role in calcium/phosphate homeostasis, it also performs other “nonclassical”2 actions that have aroused interest in the critical care field. Critically ill patients are at higher risk of having hypovitaminosis D due to various circumstances2: reduced endogenous synthesis, deficient oral intake, increased tissue requirements, impaired liver and kidney hydroxylation, and malabsorption due to intestinal oedema.
Vitamin D deficiency has been associated with increased severity in adult patients, evinced by severity scoring systems such as SOFA or PELOD.3–5 The level of 25-hydroxyvitamin D [25(OH)D] at ICU admission has been associated with overall survival in the short and long term, the duration of mechanical ventilation (MV), cardiovascular involvement and sepsis in adult patients.6–8 However, there are still few studies on its prevalence and impact on outcomes and severity in paediatric critical patients.9–12 Our objective was to determine the prevalence of VDD in an Andalusian PICU, identify the factors associated with VD levels at admission, and analyse its association with morbidity, mortality and length of stay in the PICU.
Materials and methodsObjectivesOur aim was to determine the prevalence of VDD and analyse its association with morbidity, mortality and length of PICU stay. Specifically, we sought to describe the clinical and sociodemographic characteristics of these patients, to analyse the association of 25(OH)D levels with the PRISM III score, and the independent association of VDD with sociodemographic and clinical characteristics, laboratory parameters for systemic inflammatory response syndrome (SIRS), morbidity and mortality.
DesignWe conducted a prospective cohort study. Patients were recruited consecutively as they were admitted to the PICU and classified as VD-deficient (<20ng/mL) or not deficient. Once all patients had been recruited, the second phase consisted of a prevalence study.
Patients and settingChildren aged 6 months to 17 years admitted to a medical-surgical PICU in a tertiary level university hospital. The exclusion criteria were: chronic renal disease, parathyroid disorder, malabsorption disorder and vitamin D supplementation.
VariablesWe collected data for age (months), weight (kg), body mass index (BMI [kg/m2]), race (white, black, Asian), underlying disease, scheduled/emergency admission; date of admission and parental educational attainment. We analysed complete blood count values, levels of 25(OH)D, parathyroid hormone (PTH), C-reactive protein (CRP), procalcitonin (PCT), total and ionised calcium, magnesium and blood gases within 48h from admission. In patients that underwent surgery under extracorporeal membrane circulation (ECMO) or with large-volume fluid resuscitation, blood samples were collected at least 12h after to prevent potential dilution effect of these interventions. We measured levels of 25(OH)D by electrochemiluminescence binding assay (Cobas® e-602, Roche, Switzerland). We recorded the length of stay in the PICU (in days), the need for MV for more than 24h, the use of vasoactive drugs (VADs), the need for prolonged antibiotherapy (>7 days), parenteral nutrition (PN) and continuous renal replacement therapy (CRRT), as well as the duration of all these treatments.
DefinitionsWe defined VDD as a level of less than 20ng/mL.2,13,14 We used an operational definition of morbidity, which included requiring MV for more than 24h, use of VADs, CRRT, antibiotherapy of more than 7 days’ duration or PN during the PICU stay.
Statistical analysisWe conducted the analysis in collaboration with an expert of the IBIMA-FIMABIS AMEC unit using the software R. We calculated the prevalence with a 95% confidence interval (CI). We have described continuous variables as mean and standard deviation or as median and interquartile range based on the normality of the data, and categorical variables as percentages with 95% CIs. We assessed the association between VD, baseline characteristics and outcome measures using the χ2 or Fisher's exact test for categorical variables, and Student's t test, ANOVA, Tukey's HSD test, the Mann–Whitney U test or the Kruskal–Wallis test as applicable. We used multivariate logistic regression to assess the association between VDD and patient characteristics and between VDD and morbidity and mortality. To determine whether 25(OH)D levels were independently associated with length of stay, morbidity and mortality, we included variables previously associated with these outcomes in the reviewed literature in the linear model, and those with P-values of less than 0.01 in the univariate analysis in the logistic regression model. We considered P-values of 0.05 statistically significant.
Sample sizeWe calculated the sample size using the described prevalence of VDD in healthy Spanish children of 51%.15 To estimate the VDD proportion with a 5.6% precision and a 95% CI, we needed a minimum of 307 patients for the second phase of the study.
For the first phase (cohort study), we calculated the sample size based on a relative risk (RR) of mortality at 90 days of 1.27. To achieve a precision of 0.20 units in the estimation of the logarithm of the RR with an asymptotic 95% CI for a two-sided test, assuming the expected RR to be 1.73 [CI (RR): 1.73*exp(−0.20); 1.73*exp(+0.20)] and that the proportion of individuals in the reference group relative to the total to be 50%, we needed to include 100 patients in the reference group and 100 patients in the experimental group, for a total of 200 patients in the study.
Ethical considerationsThe study protocol was approved by the competent regional research ethics committee. We provided an informational leaflet to the families and obtained their informed consent in every instance before including patients in the study. Data were recorded on an anonymous basis in an encrypted electronic database. The project adhered to the principles of the Declaration of Helsinki and standards for good clinical practice.
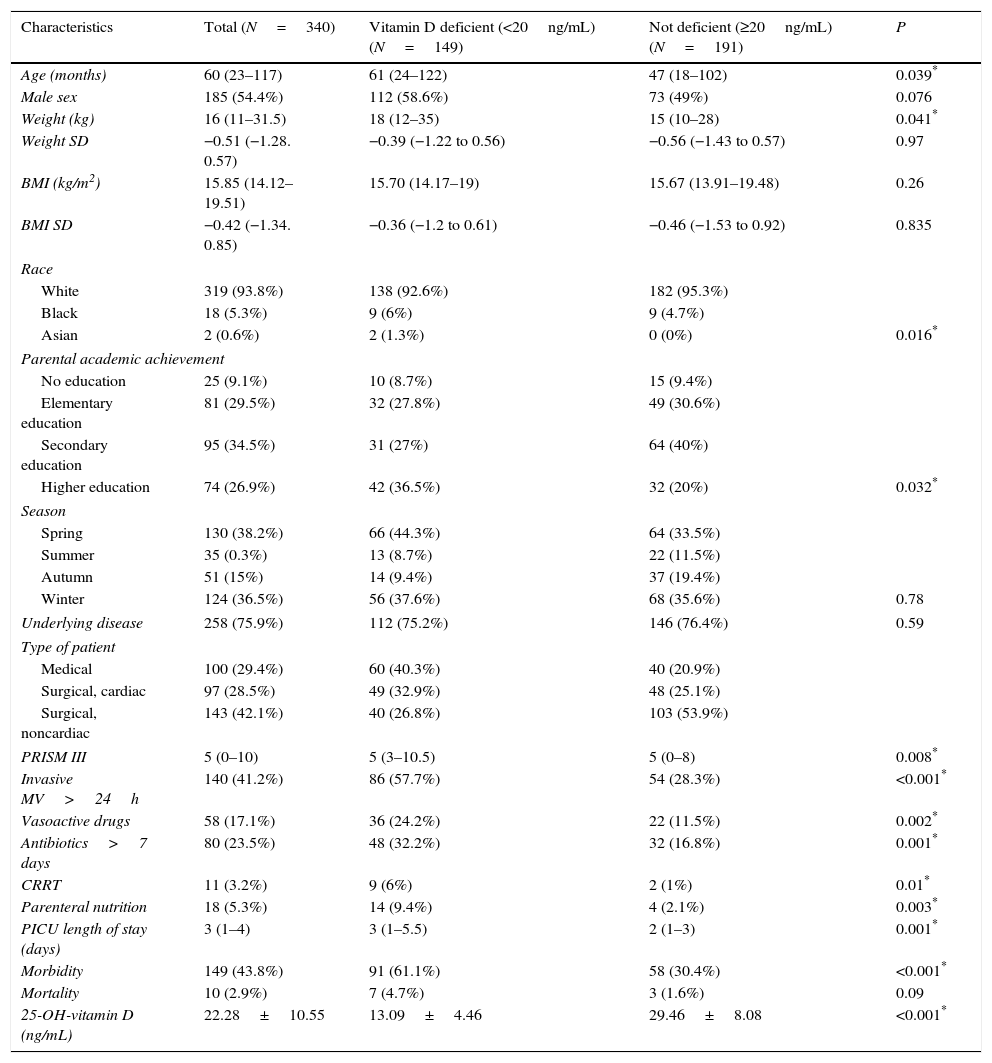
ResultsBaseline characteristicsDuring the period under study, 615 patients were admitted, of who 466 met the inclusion criteria. Out of the latter, we did not obtain a signed informed consent for 126, so the final sample consisted of 340 patients. Table 1 shows the sociodemographic and baseline characteristics of the patients.
Baseline and sociodemographic characteristics of the sample.
| Characteristics | Total (N=340) | Vitamin D deficient (<20ng/mL) (N=149) | Not deficient (≥20ng/mL) (N=191) | P |
|---|---|---|---|---|
| Age (months) | 60 (23–117) | 61 (24–122) | 47 (18–102) | 0.039* |
| Male sex | 185 (54.4%) | 112 (58.6%) | 73 (49%) | 0.076 |
| Weight (kg) | 16 (11–31.5) | 18 (12–35) | 15 (10–28) | 0.041* |
| Weight SD | −0.51 (−1.28. 0.57) | −0.39 (−1.22 to 0.56) | −0.56 (−1.43 to 0.57) | 0.97 |
| BMI (kg/m2) | 15.85 (14.12–19.51) | 15.70 (14.17–19) | 15.67 (13.91–19.48) | 0.26 |
| BMI SD | −0.42 (−1.34. 0.85) | −0.36 (−1.2 to 0.61) | −0.46 (−1.53 to 0.92) | 0.835 |
| Race | ||||
| White | 319 (93.8%) | 138 (92.6%) | 182 (95.3%) | |
| Black | 18 (5.3%) | 9 (6%) | 9 (4.7%) | |
| Asian | 2 (0.6%) | 2 (1.3%) | 0 (0%) | 0.016* |
| Parental academic achievement | ||||
| No education | 25 (9.1%) | 10 (8.7%) | 15 (9.4%) | |
| Elementary education | 81 (29.5%) | 32 (27.8%) | 49 (30.6%) | |
| Secondary education | 95 (34.5%) | 31 (27%) | 64 (40%) | |
| Higher education | 74 (26.9%) | 42 (36.5%) | 32 (20%) | 0.032* |
| Season | ||||
| Spring | 130 (38.2%) | 66 (44.3%) | 64 (33.5%) | |
| Summer | 35 (0.3%) | 13 (8.7%) | 22 (11.5%) | |
| Autumn | 51 (15%) | 14 (9.4%) | 37 (19.4%) | |
| Winter | 124 (36.5%) | 56 (37.6%) | 68 (35.6%) | 0.78 |
| Underlying disease | 258 (75.9%) | 112 (75.2%) | 146 (76.4%) | 0.59 |
| Type of patient | ||||
| Medical | 100 (29.4%) | 60 (40.3%) | 40 (20.9%) | |
| Surgical, cardiac | 97 (28.5%) | 49 (32.9%) | 48 (25.1%) | |
| Surgical, noncardiac | 143 (42.1%) | 40 (26.8%) | 103 (53.9%) | |
| PRISM III | 5 (0–10) | 5 (3–10.5) | 5 (0–8) | 0.008* |
| Invasive MV>24h | 140 (41.2%) | 86 (57.7%) | 54 (28.3%) | <0.001* |
| Vasoactive drugs | 58 (17.1%) | 36 (24.2%) | 22 (11.5%) | 0.002* |
| Antibiotics>7 days | 80 (23.5%) | 48 (32.2%) | 32 (16.8%) | 0.001* |
| CRRT | 11 (3.2%) | 9 (6%) | 2 (1%) | 0.01* |
| Parenteral nutrition | 18 (5.3%) | 14 (9.4%) | 4 (2.1%) | 0.003* |
| PICU length of stay (days) | 3 (1–4) | 3 (1–5.5) | 2 (1–3) | 0.001* |
| Morbidity | 149 (43.8%) | 91 (61.1%) | 58 (30.4%) | <0.001* |
| Mortality | 10 (2.9%) | 7 (4.7%) | 3 (1.6%) | 0.09 |
| 25-OH-vitamin D (ng/mL) | 22.28±10.55 | 13.09±4.46 | 29.46±8.08 | <0.001* |
BMI, body mass index; CRRT, continuous renal replacement therapy; MV, mechanical ventilation; SD, standard deviation (for weight and BMI based on the Fernández et al. growth charts [2011]).
The mean 25(OH)D level for the overall sample was 22.28±10.55ng/mL (95% CI, 21.15–23.41). The prevalence of VD deficiency was 43.8% (95% CI, 38.53%–49.07%). One hundred and twenty-eight patients (37.6%) had VD insufficiency (20–30ng/mL), 41 patients (12.1%) had levels between 30 and 40ng/mL, and only 22 (6.5%) had optimal levels (>40ng/mL).
Risk factors for vitamin D deficiencyThe bivariate analysis (Tables 1 and 2) found that patients with VDD were older, were admitted more frequently in the spring, had higher PRISM III scores and stayed longer in the PICU. In spring, the mean 25(OH)D level was 20.25±9.7ng/mL, while in autumn it was 26.9±11.7ng/mL. The highest level of 25(OH)D corresponded to August (32.42ng/mL) and the lowest to April (17.1ng/mL). We found a statistically significant association between parental educational attainment and VDD. The mean 25(OH)D levels for children of parents with no education, an elementary education, a secondary education and a higher education were 24.9, 22.7, 22.6 and 19.6ng/mL, respectively. Using bivariate logistic regression, we found that the risk of having VDD was greater in patients whose parents had a higher education compared to patients whose parents had an elementary education (OR, 0.498; 95% CI, 0.26–0.94) or a secondary education (OR, 0.37; 95% CI, 0.2–0.7).
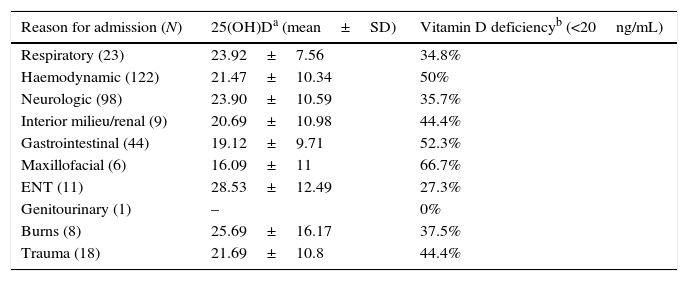
Vitamin D levels and prevalence of deficiency by reason for admission.
| Reason for admission (N) | 25(OH)Da (mean±SD) | Vitamin D deficiencyb (<20ng/mL) |
|---|---|---|
| Respiratory (23) | 23.92±7.56 | 34.8% |
| Haemodynamic (122) | 21.47±10.34 | 50% |
| Neurologic (98) | 23.90±10.59 | 35.7% |
| Interior milieu/renal (9) | 20.69±10.98 | 44.4% |
| Gastrointestinal (44) | 19.12±9.71 | 52.3% |
| Maxillofacial (6) | 16.09±11 | 66.7% |
| ENT (11) | 28.53±12.49 | 27.3% |
| Genitourinary (1) | – | 0% |
| Burns (8) | 25.69±16.17 | 37.5% |
| Trauma (18) | 21.69±10.8 | 44.4% |
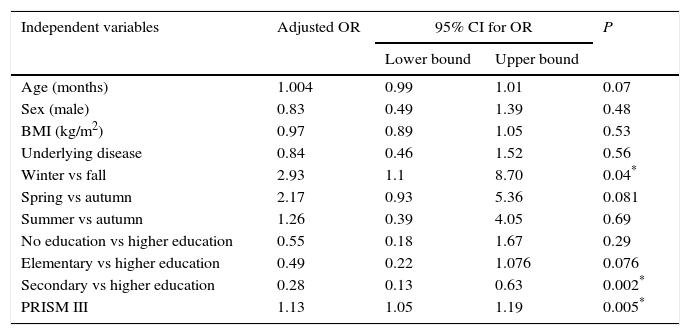
The multivariate analysis (Table 3) found an increased risk of VDD in patients admitted during winter compared to autumn (OR, 2.93; 95% CI, 1.1–8.7; P=.04) and in patients that scored higher in the PRISM III scale (OR, 1.07; 95% CI, 1.02–1.13; P=0.005).
Multivariate logistic regression model studying the risk of vitamin D deficiency (yes/no) based on the baseline characteristics of participating patients.
| Independent variables | Adjusted OR | 95% CI for OR | P | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Age (months) | 1.004 | 0.99 | 1.01 | 0.07 |
| Sex (male) | 0.83 | 0.49 | 1.39 | 0.48 |
| BMI (kg/m2) | 0.97 | 0.89 | 1.05 | 0.53 |
| Underlying disease | 0.84 | 0.46 | 1.52 | 0.56 |
| Winter vs fall | 2.93 | 1.1 | 8.70 | 0.04* |
| Spring vs autumn | 2.17 | 0.93 | 5.36 | 0.081 |
| Summer vs autumn | 1.26 | 0.39 | 4.05 | 0.69 |
| No education vs higher education | 0.55 | 0.18 | 1.67 | 0.29 |
| Elementary vs higher education | 0.49 | 0.22 | 1.076 | 0.076 |
| Secondary vs higher education | 0.28 | 0.13 | 0.63 | 0.002* |
| PRISM III | 1.13 | 1.05 | 1.19 | 0.005* |
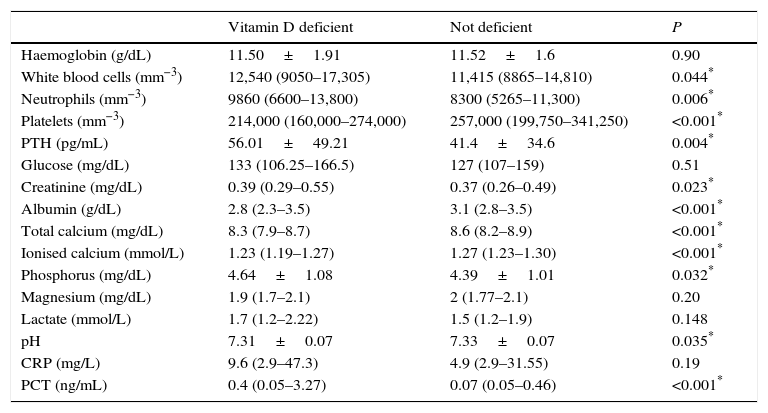
We also found an association between 25(OH)D levels and several laboratory parameters (Table 4). Patients with VDD had higher white blood cell and neutrophil counts, lower platelet counts, lower levels of albumin, ionised calcium, lower pH, and higher serum phosphate and PCT levels. The mean PTH level was 56±49pg/mL in patients with VDD and 414±34.6pg/mL in patients without VDD (P=0.004). Forty-one patients (27.5%) in the VDD group had hyperparathyroidism (PTH>60pg/mL), compared to 22 (12.6%) in the group without VDD (P=0.001). We found a negative linear correlation of PTH levels with both 25(OH)D levels (Pearson's r, −0.22; R2, 0.047; P<0.001) and ionised calcium levels (Spearman's ρ, −0.33; R2, 0.18; P<0.001).
Laboratory parameters in children with and without vitamin D deficiency.
| Vitamin D deficient | Not deficient | P | |
|---|---|---|---|
| Haemoglobin (g/dL) | 11.50±1.91 | 11.52±1.6 | 0.90 |
| White blood cells (mm−3) | 12,540 (9050–17,305) | 11,415 (8865–14,810) | 0.044* |
| Neutrophils (mm−3) | 9860 (6600–13,800) | 8300 (5265–11,300) | 0.006* |
| Platelets (mm−3) | 214,000 (160,000–274,000) | 257,000 (199,750–341,250) | <0.001* |
| PTH (pg/mL) | 56.01±49.21 | 41.4±34.6 | 0.004* |
| Glucose (mg/dL) | 133 (106.25–166.5) | 127 (107–159) | 0.51 |
| Creatinine (mg/dL) | 0.39 (0.29–0.55) | 0.37 (0.26–0.49) | 0.023* |
| Albumin (g/dL) | 2.8 (2.3–3.5) | 3.1 (2.8–3.5) | <0.001* |
| Total calcium (mg/dL) | 8.3 (7.9–8.7) | 8.6 (8.2–8.9) | <0.001* |
| Ionised calcium (mmol/L) | 1.23 (1.19–1.27) | 1.27 (1.23–1.30) | <0.001* |
| Phosphorus (mg/dL) | 4.64±1.08 | 4.39±1.01 | 0.032* |
| Magnesium (mg/dL) | 1.9 (1.7–2.1) | 2 (1.77–2.1) | 0.20 |
| Lactate (mmol/L) | 1.7 (1.2–2.22) | 1.5 (1.2–1.9) | 0.148 |
| pH | 7.31±0.07 | 7.33±0.07 | 0.035* |
| CRP (mg/L) | 9.6 (2.9–47.3) | 4.9 (2.9–31.55) | 0.19 |
| PCT (ng/mL) | 0.4 (0.05–3.27) | 0.07 (0.05–0.46) | <0.001* |
Values expressed as mean±standard deviation or median (interquartile range).
The bivariate analysis (Table 1) showed that children with VDD stayed longer in the PICU. However, in the multivariate analysis adjusted for age, PRISM III score, morbidity and reason for admission we did not find a statistically significant association between VDD and PICU length of stay.
MorbidityThe multivariate analysis found an increased morbidity in patients with VDD (Table 1). The percentage of patients requiring more than 24 hours of MV was higher in the VDD group, as was the total duration of MV. Patients with VDD required VADs more frequently and for longer periods (median, 3 vs 2 days; mean, 7.5 vs 3.05 days, P=0.24), although the difference was not statistically significant. The need for CRRT was more frequent in patients with VDD, as were prolonged antibiotherapy and the use of PN.
In the VDD group, patients with secondary hyperparathyroidism had increased morbidity compared to patients with PTH levels of less than 60pg/mL (65.9% vs 59.3%; P<0.001).
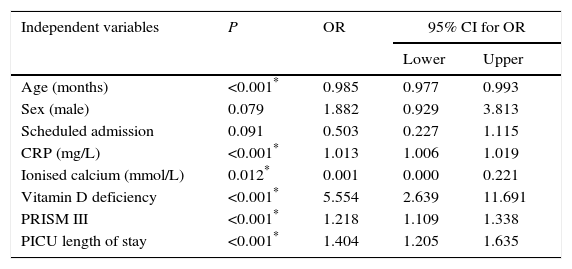
In the multivariate logistic regression analysis, the adjusted OR for VDD was 5.55 (95% CI, 2.64–11.69). C-reactive protein levels, the PRISM III score and the PICU length of stay were also associated with an increased risk of morbidity, while ionised calcium levels and age had a protective effect (Table 5).
Multivariate logistic regression analysis. Dependent variable: morbidity.
| Independent variables | P | OR | 95% CI for OR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (months) | <0.001* | 0.985 | 0.977 | 0.993 |
| Sex (male) | 0.079 | 1.882 | 0.929 | 3.813 |
| Scheduled admission | 0.091 | 0.503 | 0.227 | 1.115 |
| CRP (mg/L) | <0.001* | 1.013 | 1.006 | 1.019 |
| Ionised calcium (mmol/L) | 0.012* | 0.001 | 0.000 | 0.221 |
| Vitamin D deficiency | <0.001* | 5.554 | 2.639 | 11.691 |
| PRISM III | <0.001* | 1.218 | 1.109 | 1.338 |
| PICU length of stay | <0.001* | 1.404 | 1.205 | 1.635 |
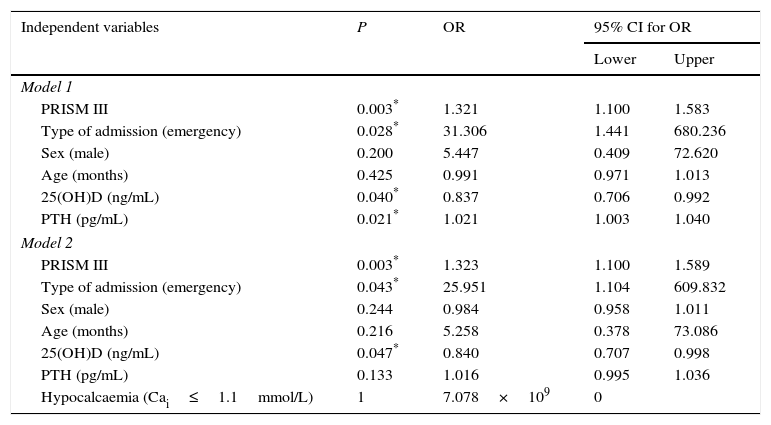
While mortality was higher in patients with VDD in the bivariate analysis, the difference was not statistically significant. Levels of 25(OH)D were lower in patients that died compared to survivors at discharge from PICU (14±8.81ng/mL vs 22.53±10.53ng/mL; P=0.012). In the multivariate regression analysis, higher levels of 25(OH)D acted as protective factors against mortality (OR, 0.84; 95% CI, 0.70–0.99; P=0.04). This effect was independent of hypocalcaemia, as shown by the second logistic regression model (Table 6). In this model, PTH levels were a risk factor for mortality (OR, 1.021; 95% CI, 1.003–1.04). However, when we included hypocalcaemia in model 2, the association of PTH with mortality was no longer significant, which suggests that calcium level is a confounding factor and potential source of bias (Table 6). The PRISM III score and emergency admission to the PICU were also associated with increased risk of mortality in the multivariate analysis (OR, 1.32 and 25.95, respectively).
Multivariate logistic regression analysis. Dependent variable: mortality.
| Independent variables | P | OR | 95% CI for OR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Model 1 | ||||
| PRISM III | 0.003* | 1.321 | 1.100 | 1.583 |
| Type of admission (emergency) | 0.028* | 31.306 | 1.441 | 680.236 |
| Sex (male) | 0.200 | 5.447 | 0.409 | 72.620 |
| Age (months) | 0.425 | 0.991 | 0.971 | 1.013 |
| 25(OH)D (ng/mL) | 0.040* | 0.837 | 0.706 | 0.992 |
| PTH (pg/mL) | 0.021* | 1.021 | 1.003 | 1.040 |
| Model 2 | ||||
| PRISM III | 0.003* | 1.323 | 1.100 | 1.589 |
| Type of admission (emergency) | 0.043* | 25.951 | 1.104 | 609.832 |
| Sex (male) | 0.244 | 0.984 | 0.958 | 1.011 |
| Age (months) | 0.216 | 5.258 | 0.378 | 73.086 |
| 25(OH)D (ng/mL) | 0.047* | 0.840 | 0.707 | 0.998 |
| PTH (pg/mL) | 0.133 | 1.016 | 0.995 | 1.036 |
| Hypocalcaemia (Cai≤1.1mmol/L) | 1 | 7.078×109 | 0 | |
Our study shows that VDD is found in nearly half of children at the time of PICU admission. Furthermore, lower 25(OH)D levels are associated with higher PRISM III scores, parental educational attainment and colder seasons.
The prevalence observed in our study was similar to the one recently reported by Madden et al.9 McNally et al.10 found a higher prevalence in a multicentric study conducted in children in Canada. A study that compared healthy children to children admitted to the PICU in Asturias conducted by Rey et al.11 also found a higher prevalence of VDD in the latter (15.6% vs 29.5%; P=0.01). Therefore, it is clear that hypovitaminosis D is a frequent condition in the PICU, although its prevalence varies widely based on sociodemographic characteristics. It is well known that VD levels decrease with increasing age in the paediatric age group.9,11,16 In our sample, the bivariate analysis found a statistically significant difference, but this difference was not maintained in the multivariate analysis, which could be due to having excluded infants aged less than 6 months. The latitude of the usual residence is also a recognised factor that influences serum 25(OH)D levels and that may partially account for the high prevalence found by McNally, as are habits associated with sun exposure, ethnicity, the time spent outdoors and VD intake.16,17 On the other hand, the method used to measure 25(OH)D levels may account for some of these differences, as has been recently described.18,19
Unlike other authors, we did not find an increased prevalence of VDD in post-operative cardiac surgery (POCS) patients.12,20 Although there are other factors involved in the underlying pathophysiology, the dilution effect resulting from ECMO that has been proposed by some authors20 may have been minimised in our study because collection of blood samples was delayed for at least 12h after admission to PICU or large-volume fluid infusion. Furthermore, not all POCS patients underwent ECMO, so the prevalence in our sample cannot be compared to those found in previous studies.12 On the other hand, we excluded infants aged less than 6 months due to the routine administration of VD for prophylaxis. Given that the operative complexity is usually greater in cardiac surgical patients aged less than 6 months, our sample is not fully representative of POCS patients overall. Thus, estimating the actual prevalence of VDD in POCS patients would require conducting a study specifically focused on these patients.
The association between higher parental educational attainment and patient VD status was an interesting finding. Spending less time outdoors, use of UVB sunscreens and sunscreen SPF, low socioeconomic status and household income have been associated with an increased prevalence of VDD.21–23 There is evidence of an association in young adults between hypovitaminosis D and high parental educational attainment.24,25 Since we did not administer a specific questionnaire to assess nutrition and daily activity, we cannot be sure about the reasons underlying this association.
In our study, the level of 25(OH)D at admission was positively correlated to platelet count, serum albumin and pH, and negatively correlated with white blood cell and neutrophil counts and serum PCT. These correlations have been reported by other authors,11,19 which suggests that VD has a significant role in SIRS and especially in sepsis.6,26,27 Procalcitonin is an important marker of host response to infection and other clinical states.28,29 Based on the differences found in laboratory parameters noted above, we hypothesise that the elevation of PCT is greater in patients with VDD because they develop a stronger inflammatory response to these various stimuli. One of the most important nonclassical effects of VD involves immunity2,6; VDD compromises the ability of the immune system to suppress bacterial infection and inflammatory responses, thus leading to increased levels of circulating PCT.30
In our study, decreased levels of 25(OH)D were associated to morbidity, and 25(OH)D concentration seemed to have a protective effect against mortality, although the number of deaths in our sample was low. These findings are plausible from a biological perspective and are consistent with those of previous clinical trials and experimental studies3,9,10,31–33 that show that VD acts as a hormone and cytokine with important pleiotropic effects on immunity and other organs with key roles in the pathophysiology of critical illness.3
Broadly speaking, VD increases serum calcium levels, which historically have been associated with morbidity and mortality.4,34 Hypocalcaemia is a frequent finding in patients with acute stress or critical illness and can lead to a compensatory rise of PTH to maintain calcium homeostasis. In our study, calcium was independently associated with a decreased risk of morbidity and VDD, but we did not find a statistically significant association with mortality. There was also no association between PTH levels and mortality after adjusting for the presence or absence of hypocalcaemia.
The increased morbidity observed in patients with VDD and secondary hyperparathyroidism compared to patients with PTH levels of less than 60pg/mL is consistent with the hypothesis proposed by Nair et al.: the lack of a PTH response at low 25(OH)D levels may result in improved tissue vitamin D utilisation, which would be associated with better disease outcomes.2,4 In addition, calcitriol levels are positively correlated with 25(OH)D levels and are up to 50% lower in critically ill patients.35 Thus, VDD in critical illness may reflect an imbalance between substrate availability and tissue requirements, in which, despite maximum stimulation of 1-α-hydroxylase by PTH, local tissue is unable to generate adequate amounts of 1,25(OH)2D.36 Thus, PTH resistance may compromise the synthesis of 1,25(OH)2D.2 The failure of PTH upregulation due to circulating VD deficiency may lead to a clinical picture of functional vitamin D insuficiency.2,36
Whether these associations reflect a causal relationship or VD levels decrease as a consequence of SIRS and increased tissue requirements has yet to be determined. Measurement of 25(OH)D levels may not accurately reflect VD status prior to the onset of illness due to SIRS and associated interventions (volume expansion, CRRT) that can decrease its serum concentration.20,27,37,38 As we observed, there is a statistically significant association between albumin levels and VDD. However, Reid et al. demonstrated that decreased serum levels of 25(OH)D after an inflammatory insult could not be explained by the levels of binding proteins and did not have an impact on adjusted calcium or PTH levels.39
The hypothesis of causality would be supported by evidence of clinical improvement with VD supplementation, which has been studied recently in adults.40 These and other aspects deserve continued investigation for the purpose of elucidating the role of VD in the pathophysiology of a variety of clinical states associated with critical illness and of designing interventions to improve outcomes in these patients.
ConclusionsVitamin D deficiency is frequent in paediatric critical patients and is associated with higher severity scores, the season of the year and parental educational attainment. Vitamin D levels were associated with various laboratory parameters of SIRS. Vitamin D deficiency is associated with an increased risk of morbidity and mortality, but our findings on its association with PICU length of stay were inconclusive. We want to highlight the relevance of assessing VD status in these patients, as well as the need for further studies to investigate the role of this vitamin in critically ill patients.
Conflict of interestsThe authors have no conflict of interests to declare.
We want to thank IBIMA-FIMABIS (Fundación Pública Andaluza para la Investigación de Málaga en Biomedicina y Salud) for the help provided in the design and statistical analysis of the study. We also thank Dr Pilar Sánchez Ocón and Dr Juan Antonio Lillo Muñoz for their guidance and for processing samples for vitamin D determination.
Please cite this article as: García-Soler P, Morales-Martínez A, Rosa-Camacho V, Lillo-Muñoz JA, Milano-Manso G. Déficit de vitamina D y morbimortalidad en pacientes críticos pediátricos. An Pediatr (Barc). 2017;87:95–103.