Early diagnosis of early-onset neonatal sepsis (EONS) is essential to reduce morbidity and mortality. Procalcitonin (PCT) in cord blood could provide a diagnosis of infected patients from birth.
ObjectiveTo study the usefulness and safety of a procedure for the evaluation of newborns at risk of EONS, based on the determination of PCT in cord blood.
Patients and methodsNeonates with infectious risk factors, born in our hospital from October 2013 to January 2015 were included. They were processed according to an algorithm based on the values of cord blood procalcitonin (<0.6ng/mL versus ≥0.6ng/mL). They were later classified as proved infection, probable, or no infection.
Results and conclusionsOf the 2.519 infants born in the study period, 136 met inclusion criteria. None of 120 cases with PCT <0.6ng/mL in cord blood developed EONS (100% negative predictive value). On the other hand, of the 16 cases with PCT ≥0.6ng/mL, 10 were proven or probably infected (62.5% positive predictive value). The sensitivity of the PCT against infection was 100%, with a specificity of 95.2% (area under the receiver operator curve 0.969). The incidence of infection in the study group was 7.4%, and 26.1% in cases with maternal chorioamnionitis. 21 newborn (15.4%) received antibiotic therapy.
The studied protocol has shown to be effective and safe to differentiate between patients with increased risk of developing an EONS, in those where the diagnostic and therapeutic approach was more interventionist, versus those with less likelihood of sepsis, who would benefit from a more conservative management.
El diagnóstico precoz es esencial para disminuir la morbimortalidad en la sepsis neonatal precoz (SNP). La procalcitonina (PCT) en sangre de cordón permitiría identificar al nacimiento a los pacientes infectados.
ObjetivoEstudiar la utilidad y seguridad de un protocolo de valoración de recién nacidos con riesgo de SNP, basado en los valores de procalcitonina en sangre de cordón.
Pacientes y métodosSe incluyeron los nacidos en nuestro hospital de octubre de 2013 a enero de 2015, con factores de riesgo infeccioso. Se procedió según un algoritmo basado en los valores de procalcitonina (<0,6ng/ml frente a ≥0,6ng/ml). Posteriormente se clasificaron como infección comprobada, probable o no infección.
Resultados y conclusionesDe 2.519 nacidos en el periodo de estudio 136 cumplieron criterios de inclusión. De 120 casos con PCT <0,6ng/ml ninguno desarrolló SNP (valor predictivo negativo 100%). Por el contrario, de 16 casos con PCT ≥0,6ng/ml, diez presentaron infección comprobada o probable (valor predictivo positivo 62,5%). La sensibilidad de la PCT frente a infección fue 100% y la especificidad 95,2% (área bajo la curva operador receptor 0,969). La incidencia de infección en el grupo de estudio fue de 7,4%; en RN de madre con corioamnionitis 26,1%. Recibieron antibioterapia 21 recién nacidos (15,4%).
El protocolo clínico estudiado ha demostrado ser efectivo y seguro para diferenciar entre pacientes con mayor riesgo de SNP, en los que la aproximación diagnóstica y terapéutica fue más intervencionista, frente a aquellos con menor probabilidad de sepsis, que se beneficiaron de un manejo más conservador.
The incidence of early-onset neonatal sepsis (EONS) has decreased drastically since the introduction of maternal prophylactic antibiotherapy.1 However, it continues to be a frequent reason for admission in neonatal units of newborns (NBs) at risk of infection, as reducing the morbidity and mortality associated with EONS early diagnosis is requiered.2
There is still no clearly defined diagnostic scheme for this condition. Its clinical manifestations are subtle and nonspecific and develop at a late stage, so it must be diagnosed while the patient is still asymptomatic. Such a diagnosis would rest on the identification of NBs at risk of early-onset sepsis, based on factors that are neither sensitive nor specific2–4 and performance of laboratory tests that are painful, reduce blood volume and are costly, and which are particularly harmful to newborns.5–7 The use of diagnostic tests is neither systematic nor definitive and requires repeated testing, including a complete blood count (CBC) with white blood cell (WBC) differential, blood culture, and determination of acute phase reactant levels, especially C-reactive protein (CPR), whose levels start to increase at 6–12h from birth in cases of sepsis.3,5,6 The use of other inflammation markers (interleukins IL-6, IL-8, IL-1 and TNF-α) has been studied with variable results and little applicability to clinical practice.8–12
A definitive diagnosis can only be obtained by a positive central sample culture. This standard is less than ideal, as it offers a low yield in the neonatal period due to various factors (small sample volumes, intermittent bacteraemia, intrapartum administration of antibiotics to the mother, etc.) and due to the delay in obtaining its results, which often results in the empirical treatment with antibiotics of patients without infection.6,13
Procalcitonin (PCT) is a prohormone secreted by most parenchymal tissues. Its usefulness as an inflammatory marker has been studied in children and adults.10–14 It exhibits a physiological elevation in healthy NBs in the first days of life that peaks at 24h; this peak is higher, occurs earlier and lasts longer in preterm NBs.14–16 Several systematic reviews have provided evidence of its usefulness in the diagnosis of neonatal infections, although its interpretation as a marker of EONS is complicated by the physiological variations that take place in the first hours of life.16–21 Recent studies demonstrate that cord blood PCT levels could be used to discriminate between infected and not-infected patients at an early stage using a cut-off point of 0.6ng/mL.22,23
ObjectiveOur aim was to analyse the usefulness and safety of a protocol for the assessment of NBs with risk factors for EONS in which the initial diagnostic and therapeutic approach would be based on cord blood PCT levels and patient symptoms.
The establishment of an algorithm could be useful in the care of NBs, so that those at low risk could be managed more conservatively compared to those at higher risk of infection and requiring a more aggressive diagnostic and therapeutic approach.
Materials and methodsWe conducted a study to assess a clinical management protocol by performing a retrospective analysis of its effectiveness and safety.
Inclusion criteria: newborns delivered at the Hospital Universitario Príncipe de Asturias in Alcalá de Henares between October 1, 2013 and January 31, 2015 with measurement of cord blood PCT levels due to the presence of the following risk factors for EONS:
- a)
Clinical suspicion of maternal chorioamnionitis diagnosed according to the Gibbs criteria,24 based on maternal intrapartum fever of more than 38°C and two or more of the following: malodorous amniotic fluid; maternal tachycardia (>120 beats per minute [bpm]); foetal tachycardia (>160bpm); maternal leukocytosis (white blood cells >15000/μL) elevated CRP in mother; maternal pelvic pain or uterine hyperstimulation.
- b)
Preterm delivery with spontaneous labour before 34 weeks gestation.
- c)
Delivery with at least 2 risk factors from the following: prolonged rupture of membranes (>18h); gestational age (GA) of less than 37 weeks; confirmed maternal colonization by Streptococcus group B (SGB) or unknown maternal SGB status with incomplete prophylaxis (less than 4h, except in cases of non-labour caesarean delivery with intact membranes) or maternal intrapartum fever.
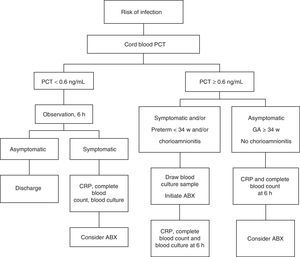
All NBs were kept under observation. Their management conformed to the algorithm presented in Fig. 1 based on cord blood PCT levels.
Asymptomatic newborns with PCT levels of less than 0.6ng/mL were kept under observation for a minimum of 48h, first in the neonatal unit and after in the maternity ward. Blood tests (CBC with WBC differential, CRP, blood culture) were only performed in symptomatic NBs. The decision to initiate antibiotherapy and to perform other diagnostic tests (lumbar puncture, chest X-ray) was based on blood cell counts and biochemistry findings. In NBs with cord blood PCT levels of 0.6ng/mL or higher, blood culture was performed at 1h from birth and early treatment initiated in those that were symptomatic or born preterm before 34 weeks gestation or with a history of maternal chorioamnionitis. Other laboratory tests (CBC with WBC differential and CRP) were performed after 6h of age, when their interpretation is more accurate.8,25–27
In all other NBs with PCT levels of 0.6ng/mL or higher, blood tests were performed at age 6h and the decision to use antibiotherapy made on a case-to-case basis. In patients that received antibiotherapy, the need for treatment was reevaluated after 48–72h based on the patient's condition and blood work results.
We assigned a final diagnosis a posteriori based on clinical, biochemical and microbiological criteria, classifying patients as cases of confirmed infection, probable infection or no infection.
We defined confirmed infection as positive isolation from a central sample (blood or cerebrospinal fluid culture) and probable infection as changes in biological or clinical manifestations with no other apparent cause in at least two clinical areas (Table 1) requiring at least 5 days of antibiotherapy. We classified NBs that did not have positive central sample cultures and did not meet any of the criteria for probable infection as not infected.
Clinical or biochemical signs of infection.
| Respiratory | Tachypnoea, apnoeas, hypoxaemia, respiratory distress |
| Cardiologic | Tachycardia (≥180bpm). Episodes of bradycardia (<80bpm) |
| Haemodynamic | Poor skin colour, peripheral hypoperfusion, hypotension without volume depletion |
| Neurologic | Irritability, lethargy or low activity |
| Gastrointestinal | Poor feeding, nutrition intolerance or abdominal distension |
| Thermoregulation | Fever (>38°C), hypothermia (<36°C) |
| Metabolic | Metabolic acidosis (base excess <−10). Hyperglycaemia (>140mg/dL) |
| Haematologic abnormalities | Leukopaenia (<5000WBC/μL) or neutropaenia (<2000neutrophils/μL) Immature/total neutrophil ratio (I/T) >0.2 Thrombocytopenia (<100000 platelets/μL) |
| Phase reactant elevation | C-reactive protein >15mg/L (6–12h) or >20mg/L from 24h |
bpm, beats per minute.
Procalcitonin tests were requested on an urgent basis and results obtained within 20min. Samples were analysed by automated chemiluminescence assay, with an accuracy of 0.02ng/mL, with the ADVIA Centaur XP® BRAMHS PCT test (Siemens Healthcare Diagnostics). We set up a threshold of 0.6ng/mL for the protocol based on the findings of Joram et al.22,23 In the statistical analysis, we described qualitative data as percentages, assessing precision by means of 95% confidence intervals (CIs), and quantitative data using percentages, medians and standard deviations.
We computed the receiver operating characteristic curves for PCT levels as well as the Youden index using the MedCalc software, determining the cut-off points as well as the sensitivity, specificity and predictive values with their corresponding CIs. We used the Excel diagnostic assessment calculator macro available at the CASPe network website (http://www.redcaspe.org). When we analysed the results, we created a composite category that included cases of confirmed and probable infection. We used Bayes’ theorem to calculate the positive post-test probability.
ResultsStudy sample: of the total of 2.519 NBs delivered in our hospital in the period under study, 153 (6%) had the specified risk factors for infection. We excluded 17 due to incorrect adherence to the study protocol. The final sample consisted of 136 NBs with risk factors for EONS that met the inclusion criteria and in whom the protocol was correctly followed.
Table 2 summarises the characteristics of the sample.
Sample characteristics.
| Total | 136 |
| Gestational age (median) | 35 weeks (95% CI, 35–36) Range, 28–41 weeks |
| Birth weight (median) | 2555g (95% CI, 2485–2619g) Range 1250–4655g |
| Maternal carriage of Streptococcus group B | 12 (8.8%) |
| Antenatal exposure to antibiotics | 66 (48.5%) |
| Maternal chorioamnionitis | 23 (16.9%) |
| Preterm birth <34 weeks gestation | 36 (26.4%), 5 chorioamnionitis |
| Other risk factors for infection | 82 (60.3%) |
Variables expressed as median with 95% confidence interval (CI), absolute frequencies and percentages.
Maternal colonisation by SGB was positive in 12 cases, negative in 52, and unknown at the time of delivery in 72. Thirty percent of mothers with chorioamnionitis were SGB carriers.
Maternal antibiotic prophylaxis was administered in 66 cases: 16 of 23 deliveries in which chorioamnionitis was suspected in the mother (5 of them at less than 34 weeks gestation); 20 of the 36 preterm patients born before 34 weeks gestation, and 35 of the remaining at-risk NBs.
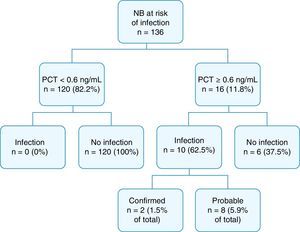
Fig. 2 summarises the final diagnoses of the patients in the study.
Cord blood PCT levels were below 0.6ng/mL in 120 NBs (82.2%), none of who developed sepsis. Procalcitonin levels were at or above 0.6ng/mL in 16 NBs (11.8%); 10 of them had infection, confirmed in 2 cases, and probable in 8. The overall incidence of infection in our study was 7.4% (confirmed in 1.5% and probable in 5.9%). Of the 16 patients with a positive PCT test, 9 (56.2%) had been born to mothers with chorioamnionitis, and 6 (37.5%) had infection.
Of all NBs with infection, only 1 was born to a mother with positive SGB status.
Twenty-one NBs (15.4%) received antibiotherapy: 7 (5.8%) of the 120 NBs with PCT levels of less than 0.6ng/mL and 14 (87.5%) of the 16 NBs with PCT levels of 0.6ng/mL or higher. None of the NBs with PCT levels of less than 0.6ng/mL that started to receive antibiotic treatment was later classified as a case of confirmed or probable infection, as they did not meet the criteria for either. Most of them (85.7%) had been born preterm (5 at GA <34 weeks) with respiratory distress, in 2 cases secondary to hyaline membrane disease and in 4 to transient tachypnoea, and there was also 1 term NB with meconium aspiration syndrome. Antibiotherapy was suspended within 4 days in all. Four out of the 6 patients whose tests were considered false positives (PCT ≥0.6ng/mL but without infection) received antibiotics: 3 born to mothers with chorioamnionitis and 1 preterm at 29 weeks gestation.
In 23 patients (16.9%) there was a history of maternal chorioamnionitis. Eleven (48%) received postnatal antibiotherapy (6 with a final diagnosis of infection and 5 born at less than 34 weeks gestation).
On the other hand, none of the 36 NBs delivered before 34 weeks gestation (26% of the sample) received a final diagnosis of infection. In 56%, the mothers had received antibiotherapy.
The distribution of the final diagnoses was: 2 cases of confirmed infection (1 by Streptococcus pneumoniae and 1 by Escherichia coli) and 8 cases of probable infection. Most NBs (92.6%) were classified as not infected. The GA of infected NBs ranged from 34 to 41 weeks. In 7 patients there was a history of maternal fever, associated with maternal chorioamnionitis in 6. Peripheral blood cultures were positive in 3. The duration of antibiotherapy in NBs classified as infected was of 6 to 14 days. Infections did not recur in any patients, and none died.
Evaluation of the diagnostic algorithmWe found no false negatives in our series: none of the 120 NBs with PCT of less than 0.6ng/mL developed EONS (negative predictive value, 100%; 95% CI, 96.9–100%). None of these patients were readmitted due to infection.
On the other hand, 10 out of the 16 NBs with PCT levels of 0.6ng/mL or higher received a diagnosis of confirmed or probable infection (positive predictive value, 62.5%; 95% CI, 38.6–81.5%), while the other 6 corresponded to false positives (4.8%).
In NBs with a history of maternal chorioamnionitis, the incidence of infection (pre-test probability) was 26.1%, while the positive post-test probability, assessed by means of Bayes’ theorem, was 88.1% (95% CI, 64.7–96.8%).
Table 3 presents the efficacy parameters of cord blood PCT for the diagnosis of infection using a cut-off point 0.6ng/mL.
Diagnostic efficacy parameters for the use of cord blood procalcitonin levels in the diagnosis of early-onset neonatal sepsis for a cut-off point ≥0.6ng/mL.
| 95% confidence interval | ||
|---|---|---|
| Sensitivity | 100% | 72.2–100% |
| Specificity | 95.2% | 90–97.8% |
| Positive predictive value | 62.5% | 38.6–81.5% |
| Negative predictive value | 100% | 96.9–100% |
| Proportion of false positives | 4.8% | 2.2–10% |
| Proportion of false negatives | 0% | 0.0–28.8% |
| Positive likelihood ratio | 21 | 9.62–45.85 |
| Negative likelihood ratio | 0 | |
| Youden index | 0.952 | |
| Pre-test probability | 7.4% |
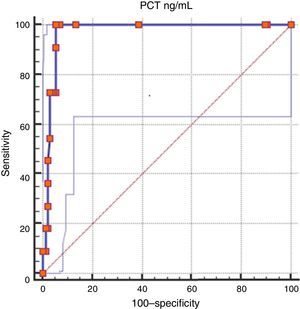
The area under the ROC curve was 0.978 (95% CI, 0.94–0.99). The Youden index for this cut-off point was 0.952 (Fig. 3).
Another 3 cases of EONS were diagnosed in our unit during the period under study, not included in the protocol because they did not meet the criteria. In two of these patients, the only risk factor was prolonged rupture of membranes of more than 24h, and in the other it was maternal bacteriuria. The total estimated incidence of sepsis in the population of newborns that received care in our hospital during the period under study was 5.2‰.
DiscussionThe first challenge in the evaluation of a diagnostic protocol for EONS is the lack of an adequate reference standard. The classification of cases with confirmed infection is unambiguous. For the diagnosis of probable infection, we developed a standard based on clinical and laboratory features similar to those used by other authors.3,4,8,11,17–21 In this type of situation, complicated by the nonspecificity of symptoms and the poor yield and delayed results of central sample cultures, the initial management protocol should be able to discriminate between infected and not infected patients at an early stage.
Due to the low incidence and the severity of EONS, it is essential to select a diagnostic test that guarantees the identification of cases of infection (high sensitivity) while ruling out infection if absent (high negative predictive value).16–18,21
Many studies have demonstrated the usefulness of PCT for the diagnosis of neonatal sepsis, with a sensitivity of 81% and a specificity of 79%.20 However, the wide variability of its levels, depending on GA and especially on the hours of life have thus far interfered with its clinical application to the early diagnosis of EONS,14–21 in which early detection and diagnostic accuracy are of the essence. This problem can be avoided by measuring PCT levels in samples of umbilical cord blood. Joram et al. found very high sensitivity and specificity values and a negative predictive value of 99% using a cut-off point of 0.6ng/mL. Cord blood PCT levels may be the key to the early diagnosis of EONS.22,25 In our study, we assessed the clinical application of these findings and obtained results that were similar and even better in sensitivity (100%), specificity (95.2%), negative predictive value (100%) and positive predictive value (62.5%). The range of patients in our sample, selected by known risk factors for infection in the immediate newborn period,1–4 was adequate, as the pre-test probability of infection was 7.4%. On the other hand, we did not find any false negatives. A similar study that was published recently obtained comparable results, although the inclusion criteria were less strict.27 Studies that have analysed other cord blood markers have found lesser sensitivity and specificity values.28–30
There are obvious advantages to the implementation in clinical practice of a protocol that can discriminate the patients at risk of infection that can be managed conservatively, as most at-risk NBs will not develop sepsis. In our study, 92.6% of NBs with risk factors for EONS did not receive a final diagnosis of infection.
On the other hand, identifying the patients at highest risk of sepsis at birth allows very early initiation of antibiotherapy in those with infection, which would hold off its progression and shorten treatment. In our study, all patients with a final diagnosis of infection received antibiotherapy from the first hour of life.
In balancing early treatment versus unnecessary treatment, measurement of cord blood PCT levels reduces the number of NBs that receive empirical antibiotherapy. It is well known that administration of broad-spectrum antibiotics in the neonatal period can have a negative impact on the intestinal microbiota, the maturation of the immune system, the risk of allergy and late-onset sepsis, bacterial drug resistance, nosocomial infection, and necrotising enterocolitis,31–34 all of which are significant iatrogenic effects.
The initial symptoms of EONS are nonspecific and may overlap with non-infectious manifestations characteristic of adaptation to extrauterine life. In NBs with PCT levels of less than 0.6ng/mL, our protocol allowed individualized decisions regarding initiation of antibiotherapy, so that a small percentage of these NBs (5.8%), most of whom had been born preterm and had respiratory problems attributed to other causes, received antibiotics at birth. Based on their outcomes, none of them met the clinical or laboratory criteria to be finally classified as cases of probable or confirmed infection. Our study did not find any false negatives, but the sample size was small, which, combined with the known small incidence of EONS, limits the power of the analysis. Further studies with a multicentric and prospective design would help validate these findings, which in turn would help prevent initiation of early empirical antibiotherapy in a greater number of preterm NBs with PCT levels of less than 0.6ng/mL.
The yield of this test is higher in children of mothers with chorioamnionitis, in who the positive post-test probability was 88.1%. Forty-eight percent of these NBs received empirical antibiotherapy, in contrast to recommendations that call for antibiotherapy in all NBs of mothers with chorioamnionitis.3,34
In our study, the positive predictive value was 62.5%, which entails a low proportion of unnecessary antibiotic treatment. It could be argued that early antibiotic treatment could prevent the development of symptoms and abnormal blood work findings in patients that would have otherwise developed clinically significant sepsis, which would have an impact on the predictive value of the test. But avoiding this bias would be difficult. It is worth nothing that none of the preterm NBs delivered before 34 weeks gestation in our series received a final diagnosis of infection; this could be due to the considerable percentage whose mothers had received antibiotic treatment before delivery (56%). Another possible conclusion would be that at present, infection has lost relevance as a factor triggering preterm labour.
We observed that the determination of PCT levels in cord blood was associated with a reduction in the number of diagnostic tests performed and of patients treated with antibiotics. It has also made it possible to identify infected patients early, and, in discriminating between NBs at higher and lower risk, allowed a reduction of the time the latter spent under observation, which favoured mother–child bonding and the establishment of breastfeeding.
ConclusionsWe found the determination of cord blood PCT levels to be useful and reliable in the assessment of NBs at risk of EONS, with a high sensitivity and specificity. Our analysis of the clinical decision-making protocol, which had limitations due to the small incidence of EONS and the sample size, showed that it was effective and safe in discriminating patients at higher risk, who were managed with more aggressive diagnostic and treatment approaches, compared to patients with a lower probability of developing sepsis, most of whom benefited from more conservative treatment, with reductions in the number of diagnostic tests, unnecessary exposure to antibiotics and length of stay.
Conflict of interestsThe authors have no conflict of interests to declare
Please cite this article as: Oria de Rueda Salguero O, Beceiro Mosquera J, Barrionuevo González M, Ripalda Crespo MJ, Olivas López de Soria C. Procalcitonina en sangre de cordón en la valoración del riesgo de sepsis neonatal precoz. An Pediatr (Barc). 2017;87:87–94.
Previous presentation: oral communication shortlisted for award at the XXV Congreso de Neonatología y Medicina Perinatal; May 2015; Seville, Spain.