Thymic regrowth after chemotherapy treatment has been reported in children with lymphoma, and in order to avoid misdiagnosing these cases as relapses, physicians should become familiar with rebound (reactive) thymic hyperplasia (RTH) and remain aware of its possible occurrence. We aimed to estimate the prevalence of RTH in children with lymphoma after completion of chemotherapy and to evaluate the clinical characteristics, outcomes, and the findings of computed tomography (CT) and gallium-67 (GA-67) scans in these patients.
Patients and methodsWe conducted a retrospective cross-sectional study by reviewing the health records of children with a lymphoma diagnosis managed at an outpatient paediatric oncology clinic in Jeddah, Saudi Arabia.
ResultsRebound thymic hyperplasia was detected in 51.9% of the lymphoma patients (14/27). It developed a median of 2.5 months after completion of chemotherapy (range, 2.0–4.25 months). Patients with RTH had significantly shorter treatment durations, and we found no significant differences between patients with and without RTH in sex, age at diagnosis, type of lymphoma or type of treatment received. All patients with RTH were asymptomatic, and routine laboratory tests did not detect any abnormalities in them. The findings of CT and GA-67 scans were highly suggestive of RTH. None of the patients with RTH had a recurrence, and RTH resolved spontaneously within a median of 6 months (range, 4.0–11.0 months).
ConclusionRTH was detected in ∼50% of children with lymphoma after completion of chemotherapy. A clinical evaluation and laboratory tests combined with imaging by CT and GA-67 can help identify RTH and rule out other lesions elsewhere.
Se ha descrito la regeneración del timo tras la quimioterapia en niños con linfoma y, para evitar diagnosticar incorrectamente estos casos como recurrencias, los facultativos han de familiarizarse con la hiperplasia tímica de rebote (HTR) y tener en consideración su posible ocurrencia. Nuestro objetivo fue estimar la prevalencia de HTR en niños con linfoma tras la quimioterapia y evaluar las características clínicas, evolución y hallazgos de las pruebas de imagen mediante tomografía computarizada (TC) y la gammagrafía con galio 67 (GA-67).
Pacientes y métodosEstudio retrospectivo transversal, mediante la revisión de las historias clínicas de niños diagnosticados de linfoma, realizado en la Clínica Ambulatoria de Oncología Infantil del Centro de Oncología de Yeda, Arabia Saudita.
ResultadosSe detectó HTR en el 51,9% de los pacientes con linfoma (14/27 pacientes). La HTR ocurrió una mediana de 2,5 meses tras finalizarse el tratamiento (rango: 2,0-4,25 meses). Los pacientes con HTR recibieron tratamientos significativamente más cortos, y no se observaron diferencias entre pacientes con y sin HTR en cuanto al sexo, la edad al diagnóstico, el tipo de linfoma o el tipo de tratamiento recibido. Todos los pacientes con HTR se encontraban asintomáticos y las pruebas rutinarias de laboratorio no evidenciaron alteraciones. La TC y la GA-67 fueron altamente sugestivas de HTR. Ninguno de los pacientes con HTR tuvieron recurrencias y la HTR se resolvió espontáneamente en una mediana de 6 meses (rango: 4,0-11,0 meses).
ConclusiónSe detectó HTR en alrededor del 50% de los niños con linfoma tras completarse el tratamiento. La evaluación clínica, pruebas de laboratorio, TC y gammagrafía con GA-67 resultan útiles para identificar la HTR y descartar otras lesiones en otras localizaciones.
The thymus is a sensitive organ that responds with atrophic changes to stress factors such as fever, starvation, chemotherapy, radiotherapy and steroid therapy. It can regain its original size or even rebound to a larger size after atrophy.1,2 Thymic regrowth after chemotherapy has been reported more often in children and adolescents compared to adults.3–6 Thymic hyperplasia has been described in children with lymphoma.7–9 Such thymic rebound or physiologic thymic regeneration following chemotherapy induces involution.10
Physicians should be made aware and become familiar with the possible development of rebound (reactive) thymic hyperplasia (RTH), as misinterpreting this phenomenon may lead to prolongation of unnecessary toxic chemotherapy or excessive exposure to radiation, and to performance of associated interventions that are not free of risk, such as biopsy, needle aspiration, or thymectomy.11–13 Although it can be challenging, it is nevertheless vitally important to differentiate RTH from a relapsed or metastatic mediastinal tumour. If RTH is misdiagnosed as a relapsed or a malignant tumour, the result is the performance of unnecessary treatment; moreover, misdiagnosis of a malignant mediastinal tumour as RTH can delay crucial life-saving therapy.7 The gold standard for diagnosis of a suspected mediastinal mass continues to be the pathological examination of a surgical biopsy specimen, which carries the risks associated with general anaesthesia and surgery. Thus, we believe it is more important to use every possible noninvasive means, such as the clinical evaluation and laboratory and imaging tests to accurately diagnose RTH in lymphoma patients after completion of chemotherapy.7
Many medical errors and misdiagnoses can be avoided by using nuclear imaging techniques, and computed tomography (CT), positron emission tomography (PET) and gallium-67 scintigraphy (GA-67) provide invaluable data to guide the crucial differential diagnosis between RTH and mediastinal tumours.7–9 Very few studies have evaluated children with lymphoma and RTH using CT,8 PET7 and GA-679 scans.
The aim of our study was to estimate the prevalence of RTH in childhood survivors of lymphoma after their treatment ended, and to document and analyse the clinical characteristics, laboratory, CT and GA-67 test results associated with the presence of RHT. We also deemed it relevant to analyse the associated risk factors and the outcomes of RTH.
Patients and methodsAfter the retrospective cross-sectional study protocol was approved by the King Abdullah Medical City (KAMC) Institutional Review Board, we collected data from the health records of paediatric survivors of lymphoma between 2008 and 2013. The patients were still being followed up at the KAMC Oncology Centre in the city of Jeddah, Saudi Arabia, as of February 2018. All the long-term follow-up paediatric patients were aged 1–18 years, had completed chemotherapy and were diagnosed with lymphoma of different types, including Hodgkin and non-Hodgkin lymphoma. We analysed the records of all patients that developed RTH after chemotherapy, retrieving data on their clinical characteristics, such as the age at diagnosis, sex, type of lymphoma, prescribed treatment and its duration, as well as the results of laboratory tests after detection of RTH, which provided relevant information such as the values of the complete blood count (CBC) and serum C-reactive protein (CRP), lactate dehydrogenase (LDH) and ferritin tests.
Rebound thymic hyperplasia was identified when an increase in thymus size relative to a previous CT scan was detected after chemotherapy. The results of GA-67 scans were also reviewed at the time RTH was identified. It was not possible to do PET scans, as they were unavailable at our site and deemed too costly.
Statistical analysisWe performed the statistical analysis with the software Statistical Package for the Social Sciences (SPSS) version 18.0 for Windows (SPSS Inc; Chicago, IL, USA). We have expressed categorical data as absolute frequencies and percentages, and quantitative data as mean±standard deviation (SD). We compared categorical data by means of the Student t and the χ2 tests, and defined statistical significance as a P-value of less than .05.
ResultsWe only found complete data for 27 of the 35 lymphoma patients eligible for the study. Table 1 summarizes the characteristics of the patients included in the final sample. Fourteen patients developed RTH after treatment, a median of 2.5 months after its completion (range, 2.0–4.25 months), which persisted for 4.0 to 11.0 months, with a median duration of 6 months (Table 2).
Characteristics of the patients.
| Overall sample27 patients (100%) | Patients with RTH14 patients (51.9%) | Patients without RTH13 patients (48.1%) | P | |
|---|---|---|---|---|
| Sex: | ||||
| Male:female | 15 (58%):12 (42%) | 4 (28.6%):10 (71.4%) | 8 (61.5%):5 (38.5%) | .09 |
| Age at diagnosis (year): | ||||
| Mean±SDMedian (IQR)[Minimum–maximum] | 6.93±3.026.0 (4.0–9.0)[3.0–12.0] | 7.0±3.356.0 (4.5–11.0)[3.0–11.0] | 6.8±2.757 (4.0–9.0)[3.0–12.0] | .84 |
| Type of lymphoma: | ||||
| Hodgkin lymphomaNon-Hodgkin lymphoma | 9 (33.3%)18 (66.7%) | 4 (28.6%)10 (64.3%) | 5 (38.5%)8 (61.5%) | .44 |
| Cancer staging: | ||||
| I–IIIII–IV | 7 (25.9%)20 (74.1%) | 3 (21.4%)11 (78.6%) | 4 (31.6%)9 (68.4%) | .75 |
| Relapse: | ||||
| YesNo | 2 (7.4%)25 (92.6%) | 014 (100%) | 2 (15.4%)11 (84.6%) | .12 |
| Steroid treatment: | ||||
| YesNo | 22 (81.5%)5 (18.5%) | 12 (85.7%)2 (14.3%) | 10 (76.9%)3 (23.1%) | .64 |
| Radiotherapy: | ||||
| YesNo | 7 (25.9%)20 (74.1%) | 2 (14.3%)12 (85.7%) | 5 (61.5%)8 (38.5%) | .20 |
| Treatment duration (months): | ||||
| Mean±SDMedian (IQR) | 9.74±2.6610.0 (8.0–12.0) | 8.71±2.788.0 (6.0–12.0) | 10.84±2.1112 (8.5–12.5) | *.035 |
IQR, interquartile range; RTH, rebound thymic hyperplasia; SD, standard deviation.
Clinical, laboratory and imaging characteristics of patients with rebound thymic hyperplasia.
| Case | Age at diagnosis, sex | Type of lymphoma, stage | Primary site of presentation | Duration of treatment, response | Time of RTH after end of treatment | Laboratory findings | Imaging findings | Outcome of RTH | Duration of follow-up |
|---|---|---|---|---|---|---|---|---|---|
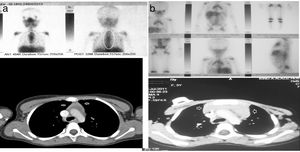
| 1 | 3 y, F | BL, stage III | Very large pelvic-abdominal mass | 6 monthsGood, CR | 3 months | LDH: 195U/LFerritin: 50ng/mLCBC and CRP: normal | CT: anterior mediastinum soft tissue isodense massGA-67: new avid lesion in the mediastinum, complete regression of old lesions in abdomen and pelvis (Fig. 1a) | Resolved in 10 months | 7 y, no relapse |
| 2 | 4.7 y, F | HD (NS) stage IB | Very large right cervical LAP | 6 monthsGood, CR | 4 months | LDH: 290U/LFerritin 31ng/mLCBC and CRP: normal | CT: homogenous mass in soft tissue of anterior mediastinumGA-67: mild uptake in mediastinum, no other lesions (Fig. 1b) | Spontaneous regression in 6 months | 6 y, no relapse |
| 3 | 11y, F | HD (NLPHD) stage IIA | left parotid, submandibular and cervical LAP. | 8 months(SER)local irradiation | 2 months | LDH:173U/L,Ferritin: 41ng/mL,CBC and CRP: normal | CT: homogenous mass in soft tissue of anterior mediastinum.GA-67: faint gallium uptake in mediastinum, no other lesions | spontaneous regression in 4 months | 5 y, no relapse |
| 4 | 5 y, F | BL, stage III | Maxillary sinus mass, pelvic-abdominal masses | 6 monthsGood, CR | 4 months | LDH:150U/LFerritin: 35ng/mLCBC and CRP: normal | CT: homogenous mass in soft tissue of anterior mediastinum.GA-67: avid lesion in mediastinum, no other lesions | Spontaneous regression in 12 months | 9 y, no relapse |
| 5 | 12 y, F | HD (NS) stage IV | Deep cervical LAP, chest, pelvic-abdominal LAP | 12 monthsGood, CR | 3 months | LDH: 134U/LFerritin: 63ng/mLCBC and CRP: normal | CT: homogenous mass in soft tissue of anterior mediastinum.GA-67: avid lesion in mediastinum, no other lesions | Persistent smaller mass, spontaneous regression (in 18 months) | 5 y, no relapse |
| 6 | 6 y, F | BL, stage IV | Generalized LAP, HSM, BM involvement | 12 monthsGood, CR | 4 months | LDH: 122U/LFerritin: 56ng/mLCBC and CRP: normal | CT: homogenous mass in soft tissue of superior mediastinumGA-67: avid lesion in mediastinum | Spontaneous regression in 4 months | 7 y, no relapse |
| 7 | 13 y, M | NHL, DLBCL, stage IIIB | Bilateral cervical LAP, very enlarged left tonsil | 8 monthsGood, CR | 14 months | Recurrent neutropenia, cellulitis, fungal and viral lung infectionsLDH: 534U/LFerritin: 663ng/mLCBC normal, CRP: 35mg/L, CD4/CD8 decreased, normal Ig | CT: anterior mediastinal enlargementGA-67: enhanced uptake in mediastinum | Unknown | 1 y, lost to follow-up immunodeficiency? |
| 8 | 4 y, F | BL, stage III | Intestinal mass and obstruction | 6 monthsGood, CR | 2 months | LDH: 140U/LFerritin: 45ng/mLCBC and CRP: normal | CT: homogenous mass in soft tissue of anterior mediastinumGA-67: mild active tracer uptake in mediastinum, no other lesions | Resolved in 12 months | 11 y, no relapse |
| 9 | 11 y, M | HD (NS), stage IIA | Bilateral cervical LAP | 6 monthsLow-dose radiation IFRT | 2 months | LDH: 102U/LFerritin: 36ng/mLCBC and CRP: normal | CT: mass in soft tissue of anterior mediastinumGA-67: avid lesion in mediastinum, no other lesions | Resolved in 9 months | 9 y, no relapse |
| 10 | 8 y, F | NHL, DLBCL stage III | Tonsillar and bilateral cervical LAP mediastinal, HSM | 6 monthsGood, CR | 2 months | LDH: 175U/LFerritin: 56ng/mLCBC and CRP: normal | CT: mass in soft tissue of anterior mediastinumGA-67: avid lesion in mediastinum, no other lesions | Spontaneous resolution in 6 months | 7 y, no relapse |
| 11 | 5 y, M | BL,stage IV | Generalized LAP | 12 monthsGood, CR | 2 months | LDH: 166U/L,Ferritin: 34ng/mL,CBC and CRP: normal | CT: oval shape, enlarged mediastinumGA-67: oval shape enhancement (warm uptake), no other lesions | Resolved in 4 months | 7 y, no relapse |
| 12 | 6.5, F | NHL, ALCL, stage IV | Fever, bone achesPelvic bone lesions | 10 monthsGood, CR | 1.5 months | LDH: 188U/L,Ferritin: 25ng/mL,CBC and CRP: normal | CT: anterior mediastinal soft tissue massGA-67: mildly increased uptake of mediastinum, no other lesions | Resolved in 3 months | 9 y, no relapse |
| 13 | 3.5 y, F | BL, stage IV | Intestinal mass, obstruction | 12 months Good, CR | 2 months | LDH: 210U/LFerritin: 49ng/mLCBC and CRP: normal | CT: anterior mediastinal soft tissue massGa-67: mildly increased uptake of mediastinum, no other lesions | Resolved in 4 months | 10 y, no relapse |
| 14 | 6 y, M | BL, stage IV | Epigastric mass, generalized LAP | 12 months Good, CR | 2 months | LDH: 193U/LFerritin: 28ng/mLCBC and CRP: normal | CT: anterior mediastinal soft tissue massGA-67: mild uptake in mediastinum, no other lesions | Resolved in 6 months | 4 y, no relapse |
ALCL, anaplastic large cell lymphoma; BL, Burkett lymphoma; BM: bone marrow; CBC, complete blood count; CR, complete response; CRP, C reactive protein; CT, computed tomography; DLBCL, diffuse large B cell lymphoma; F, female; GA-67, gallium 67 scintigraphy; HD, Hodgkin disease; HSM, hepatosplenomegaly; IFRT, involved field radiotherapy; Ig, immunoglobulins; LAP, lymphadenopathy; LDH, lactic dehydrogenase; M, male; NHL, non-Hodgkin lymphoma; NLPHD, nodular lymphocyte predominant Hodgkin lymphoma; NS, nodular sclerosis; RTH, rebound thymic hyperplasia; SER, slow early response; y, year.
The duration of chemotherapy had been shorter in patients with RTH compared to patients without RTH. We found no significant differences between patients with and without RTH in the other variables, including sex, age at diagnosis, lymphoma type or stage, and radiotherapy/steroid treatment. There were also no significant differences in the time elapsed between completion of treatment and development of RTH or the duration of RTH. All patients with RTH were asymptomatic and presented without fever, weight loss, excessive sweating or thoracic symptoms such as respiratory distress, cough, shortness of breath, chest pain or chest pressure. The clinical evaluation and physical examination of the thorax were normal in all patients with RTH. There was also no evidence of lymphadenopathy or of enlargement of abdominal organs or masses on palpation.
At the time of RTH detection, the findings of laboratory tests were all normal, including the CBC, white blood cell differential and serum levels of CRP, LDH and ferritin. Only 1 patient (patient 7 in Table 2) exhibited recurrent neutropenia and viral/fungal chest infections for a long period of time, and immunodeficiency was suspected, but unfortunately the patient dropped out of follow-up and his outcome is unknown. In this sample, no invasive procedures were required for diagnosis of RTH. In all patients with RTH patients, the CT scan revealed homogeneous anterior mediastinal enlargement at the level of the thymus with its characteristic shape—an oval or triangular mass, although in a single patient the thymus was bilobed—with no other masses or evidence of enlarged lymph nodes elsewhere. The GA-67 scans showed a homogeneous pattern of increased uptake in the anterior mediastinum, with no additional uptake in the lymph nodes or in other lesions, as illustrated by Fig. 1a and 1b, which correspond to the patients 1 and 2 in Table 2. In all patients, the GA-67 scans showed increased uptake by the thymus that disappeared in follow-up scans. Most importantly, the absence of uptake in the lymph nodes or other areas in the CT and GA-67 scans was highly suggestive of RTH, which was confirmed by the regression of the enlarged thymus in subsequent imaging scans. Rebound thymic hyperplasia spontaneously regressed and resolved in 4 to 11 months, at a median of 6 months. None of the patients with RTH experienced a relapse over the long-term follow-up of 5–9 years.
(a and b) CT and GA-67 scan images of two rebound thymic hyperplasia (RTH) patients, corresponding to patient numbers 1 and 2 from Table 2.
True thymic hyperplasia is a rebound phenomenon characterized by an increase in mass of the thymus, both in size and weight, in response to a stressor such as steroid treatment, chemotherapy, irradiation, burns and/or surgery.14 The thymus may maintain its normal bilobate shape or, more frequently, present an oval appearance.15,16 When the body experiences stress, and depending on its duration and severity, the thymus can shrink to up to approximately 40% of its original size.17 Once the body recovers, the thymus usually regains its original size within 9 months, and may even become larger (by >50%) in the phenomenon known as RTH.17,18 Rebound thymic hyperplasia is more frequent in younger children, but it can happen at all ages in children as well as adults.19
In our study, we found RTH after completion of chemotherapy in 14 of the paediatric patients suffering from different types of lymphoma (51.9%). Hyperplasia developed between and 2.0–4.25 months after treatment, at a median of 2.5 months, and persisted for 4–11 months. Our findings were similar to those of previous studies,9 which have reported development of RTH occurring after completion of chemotherapy in 67.7% of patients (21/31 subjects) over a period of 1 to 17 months, lasting 3 months to 5 years and regressing gradually over time.9Table 3 lists major past studies that have analysed RTH in children with lymphoma.
Main research studies that described RTH in children with lymphoma.
| Study, country | Studied patients, age at diagnosis | Evaluation/imaging | Findings | Conclusions |
|---|---|---|---|---|
| Our Study (2018), Saudi Arabia | 27 children, median 6.0 y (range, 4.0–9.0) | Clinical evaluation, laboratory tests, CT scan after end of treatment followed by GA-67 scan | RTH in 14/27 (51.9%)RTH developed a median of 2.5 months (range, 2.0–4.25) after completing treatment.CT scan: homogenous mass in anterior mediastinum.GA-67 was positive in all cases (14/14).All patients with RTH were asymptomatic with normal LDH, ferritin, CBC, and CRP.Spontaneous resolution or decrease in thymus size within 4–11 months in all patients, without relapse after up to 9 years’ follow-up. | RTH should be carefully evaluated (clinical, assessment, laboratory tests, CT imaging and GA-67 scan) to avoid misinterpretation as relapse and unneeded biopsies or treatments. |
| Chen et al. (2017), Taiwan9 | 31 patients (out of 67 with lymphoma)Age 3–18 y (median 10) | CT scan after complete remission, GA-67 and PET scans | RTH in 67.7% (21/31 patients)Detected 1–17 months after the end of chemotherapy, persisted 3 months-5 years and regressed gradually.All patients with RTH were asymptomatic.GA-67 scans were positive in 45% (9/20 patients with RTH).PET with increased uptake in 66.7% (4/6 patients). | No patients with RTH had lymphoma relapse during follow-up, which lasted a median of 5 years.Awareness of RTH is important to avoid unnecessary surgery/chemotherapy. |
| Tian et al. (2015), China8 | 52 lymphoma childrenMedian follow-up 32.9 months (range, 10.5–89.4) | Clinical evaluation and CT scan | Mediastinal masses detected after a median of 8.6 months from complete remission.All patients had a single mediastinal mass; in 81% a homogeneous density with clearly defined margins (trapezoidal or triangular shapes); 19% diffuse shapes with poorly defined margins. Mild enhancement after contrast in all masses.81% of masses shrank naturally, and 19% remained unchanged (after 10.5–73.9 months’ follow-up) | RTH often occurs in children with lymphoma receiving chemotherapy (regardless of whether the tumour initially invades the mediastinum or not). |
| Zhen et al. (2010), China7 | 26 children and adolescents with RTH | CT scan then PET scan or close clinical follow-up | RTH detected after a mean of 4 months (range, 1–12 months) after last chemotherapy cycle.CT: diffusely enlarged thymic parenchymal tissue with normal morphology.PET (20 patients):5 (25%) no uptake, and 15 (75%) regular shape and uniform radioactivity (false-positive results).RTH: SUV usually <4, regular morphology and uniform radioactivity distribution. Malignant tumours: SUV >10, irregular morphology with heterogeneous radioactivity distribution.All patients survived and remained disease-free (mean duration of follow-up, 40 months). | RTH following chemotherapy can be diagnosed with clinical evaluation combined with CT and PET scans (without a histopathological diagnosis). |
CT, computed tomography; GA-67, gallium-67 scintigraphy; PET, positron-emission tomography; RTH, reactive thymic hyperplasia; SUV, standardized uptake value.
We found that RTH was more prevalent in female patients, but this difference was not significant (P=.09). Chen et al.9 reported that the average age of RTH patients was lower compared to patients without RTH and that female patients were more likely to develop RTH than their male peers.
We found that chemotherapy had lasted significantly less in patients that developed RTH, which may explain its occurrence soon after they had completed treatment, after 2.0–4.25 months. A possible explanation for this finding is that patients that receive longer treatments experience longer suppression, which would prevent the development of RTH early enough to be detected in post-treatment imaging tests.
The time elapsed between completion of chemotherapy and detection of RTH in our study ranged between 2.0 and 4.25 months. Furthermore, thymic hyperplasia persisted for 4 to 11 months and gradually regressed, which was consistent with the findings of previous studies.9 All our paediatric patients with RTH were asymptomatic, in agreement with the previous literature.9 We also found no abnormalities in the laboratory test results of patients with RTH, including the CBC and measurements of serum levels of CRP, LDH and ferritin. The findings of the CT scan of the thymus were interpreted as benign, with smooth homogeneous enlargement and no other lesions elsewhere. The GA-67 scan confirmed mild-to-moderate uptake in the thymus in patients with RTH in the absence of any other lesions elsewhere, which is suggestive of RTH.
In our study, thymic enlargement was usually misinterpreted as relapsing lymphoma, but the absence of clinical signs and symptoms, and the normal results of laboratory tests and CT and GA-67 scans quickly suggested that the condition was benign. None of our patients with RTH relapsed even after long periods of follow-up, which lasted up to 9 years in some patients. In patients with a known history of malignancy, it was very challenging to diagnose RTH, as it was necessary to differentiate this mass from recurrent/metastatic mediastinal tumours. Detection of size increases in a mediastinal mass would exacerbate concerns regarding the likelihood of a potential relapse. Rebound thymic hyperplasia typically presents as a diffuse enlargement with a smooth contour, a non-lobulated mass of fine, mixed lymphoid and fat tissue with normal vasculature and no mass effect on the surrounding structures. In comparison, thymic tumours have a nodular contour and often contain foci of haemorrhage, necrosis or calcification.18,19
None of the patients with RHT in our sample relapsed, and we found no significant differences between them and patients without RTH, which was consistent with the findings of other authors, including Chen et al.,9 who found a decreased chance of relapse among RTH patients for reasons yet unknown. We found the same and have no explanation for the absence of relapse in patients with RTH, although it may be due to or associated with the recovery or activity of the immune system that triggers RTH, a phenomenon that requires further investigation.
Invasive procedures were not needed to diagnose RTH, as our patient were asymptomatic and had normal results of blood tests (CBC and serum CRP, LDH, and ferritin levels). Rebound thymic hyperplasia spontaneously regressed and resolved fully during the long-term follow-up.
In our study, we assessed children with lymphoma by means of GA-67 and CT. Unfortunately, we were unable to assess them with PET scans, as it was deemed too costly and was unavailable in our entire region. Scintigraphy with GA-67 is very useful for functional imaging of lymphomas at the time of the initial diagnosis (staging) and to assess the response to treatment.20 However, GA-67 imaging also has some clear limitations, such as the negative impact of the location of the lymphoma on sensitivity and specificity, leading to inaccuracies in the assessment of infradiaphragmatic disease. Also, the sensitivity of this technique declines in low- or intermediate-grade lymphomas. This is compounded by the need to wait a longer interval, of at least 3 days, between the injection of gallium and performance of the test just to get rid of the background activity.21 PET scan is a functional imaging technique that detects malignant cells due to their higher glycolytic activity compared to normal cells.22 In children with lymphoma, PET scans have been found to perform better than GA-67 scans, as they can differentiate scar tissue from residual lymphoma at the end of treatment. Compared to CT, MRI and GA-67, PET has shown a higher and excellent sensitivity and specificity.23,24 However, increased glycolysis caused by benign conditions, such as inflammation induced by surgery, chemotherapy, radiotherapy, infection, or granulomatous disease, may lead to false-positive PET scans due to increased uptake, especially when scans are taken immediately after these triggers.25,26 Also, we ought to keep in mind that there is evidence that PET scans cannot differentiate between thymic hyperplasia and infiltration of the thymus by malignant cells.27
Rebound thymic hyperplasia is a physiological phenomenon that may theoretically happen in any patient with lymphoma after completion of chemotherapy. It is vitally important that oncologists become fully aware of this phenomenon to avoid misinterpreting it as a relapse, which could result in the performance of unnecessary surgical interventions, such as biopsies, and/or cause undue fear and distress to both patients and their families with the news of a suspected relapse. We suggest that if mediastinal enlargement or widening is discovered in a paediatric lymphoma patient after completion of treatment, the routine follow-up should include a thorough clinical and laboratory assessment as well as a physical examination to search for any suspicious abnormalities. Chest CT scans and GA-67 may be helpful in assessing the nature of thymic enlargement while exploring the potential presence of additional masses or lymph node involvement. Rebound thymic hyperplasia should be strongly suspected in case of homogenous enlargement of the thymus in the absence of other abnormalities, a finding that should in itself be considered an indication for close monitoring and follow-up. However, if evidence is found of additional lymph node enlargement, further thymic abnormalities or masses close to this region, a biopsy should be performed immediately to rule out a relapse of lymphoma.
In our centre, the approach used was performance of GA-67 and CT scans in combination with a careful clinical assessment and laboratory tests, reinforced by close follow-up of the patients. This was considered very helpful and may be more useful for paediatric oncologists in evaluating children with suspected RTH, especially in developing countries with limited access to imaging facilities or surgical interventions.
Limitations of the study: retrospective study conducted in a single oncology centre with a relatively small number of patients with lymphoma, patients were not assessed with PET scans.
Conflict of Interest StatementThe authors declare that they have no conflicts of interest.
Please cite this article as: Fouda A, Kandil S, Hamid G, Boujettif K, Mahfouz M, Abdelaziz M. Hiperplasia tímica de rebote posquimioterapia en niños con linfoma. An Pediatr (Barc). 2019;91:189–198.