Hypoxic-ischaemic encephalopathy is a clinical syndrome of neurological dysfunction that occurs immediately after birth following an episode of perinatal asphyxia. We conducted a scoping review to assess the methodological quality of clinical practice guidelines that address this condition.
MethodologyWe conducted the evaluation using the AGREE II tool. High methodological quality was defined as a score greater than 70% in every domain.
ResultsThe analysis included three clinical practice guidelines; the highest scores were in the scope and purpose domain (84.26%; SD, 14.25%) and the clarity of presentation domain (84.26%; SD, 17.86%), while the lowest score corresponded to the applicability domain (62.50%; SD, 36.62%). Two guidelines were classified as high quality and one guideline as low-quality.
ConclusionsTwo of the assessed guidelines were classified as being of high quality; however, the analysis identified shortcomings in the applicability domain, in addition to methodological variation between guidelines developed in middle- or low-income countries versus high-income countries. Efforts are needed to make high-quality guidelines available to approach the management of hypoxic-ischaemic encephalopathy in newborns.
La encefalopatía hipóxica-isquémica (EHI) es un síndrome clínico de disfunción neurológica que se presenta inmediatamente después del nacimiento, tras un episodio de asfixia perinatal. Se llevó a cabo una revisión de alcance con el objetivo de evaluar la calidad metodológica de las guías de práctica clínica referente a esta patología.
MetodologíaLa evaluación se realizó mediante la herramienta AGREE II. El criterio de alta calidad metodológica se definió si todos los dominios obtuvieron una puntuación superior al 70%.
ResultadosSe incluyeron 3 guías de práctica clínica, las puntuaciones más altas fueron en los dominios de "alcance y objetivo" (84,26%, SD = 14,25) y "claridad de presentación" (84,26%, SD = 17,86), mientras que el dominio más bajo fue "aplicabilidad" (62,50%, SD = 36,62). Dos guías fueron catalogadas como de "alta calidad" y una guía como de "baja calidad".
ConclusionesDos guías analizadas fueron catalogadas como de alta calidad; sin embargo, se encontraron carencias en el dominio de aplicabilidad, además de variación metodológica entre guías elaboradas en países de ingresos medios o bajos y países con ingresos altos. Se requiere trabajar arduamente para proporcionar guías de alta calidad para abordar el manejo de la encefalopatía hipóxico isquémica en recién nacidos.
Hypoxic-ischaemic encephalopathy (HIE) is a neurological syndrome that presents immediately after birth following a perinatal episode of asphyxia that can cause brain damage and disability.1–4
At the global level, based on a report by Lawn et al., the main direct causes of neonatal death are preterm birth (28%), severe infections (26%) and neonatal asphyxia (23%).5 In high-income countries, the incidence of HIE is approximately 1–3 cases per 1000 births.4 In Latin America and the Caribbean, the incidence varies between countries depending on the available resources and living conditions, but there are no data allowing a description of the current situation in the region.1 In Ecuador, according to the Instituto Nacional de Estadísticas y Censos (National Institute of Statistics and Population Surveys), perinatal asphyxia was the sixth leading cause of child mortality as of 2016, and a clinical practice guideline (CPG) for the diagnosis and management of HIE has been available since 2019.2
Clinical practice guidelines promote interventions based on the evidence of highest quality currently available and can contribute to reducing morbidity and mortality and improving patient quality of life in addition to guiding clinical decision-making.6,7 Therefore, it is essential that their development is held to rigorous standards to ensure that it yields the best possible recommendations, integrating the perspective of patients and taking into account existing health care challenges. In the case of newborns with HIE, this is particularly important given its potentially severe and irreversible neurologic sequelae, which constitute a significant social and public health problem.
Different international studies have contributed evidence on strategies to reduce the incidence of neonatal asphyxia and HIE,4–6 one of which is therapeutic hypothermia (TH), which is the current standard of care for moderate to severe HIE in high-income settings10; however, it is still uncertain whether this practice is fully effective in middle- to low-income countries.11,12 This underscores the need to analyse factors outside CPGs that have an impact on the implementation of existing recommendations.
At the international level, we found a study similar to ours that, after evaluating the CPGs on HIE, only recommended one document for use in neonatal care practice on account of its methodological quality3; however, the study only included guidelines in English and Arabic, so we felt the need to address this gap by evaluating guidelines in the Spanish language. We chose to perform a scoping review due to the need to systematically explore and map national and international CPGs for HIE with the aim of assessing the methodological quality of the guidelines by means of the Appraisal of Guidelines for REsearch & Evaluation II (AGREE II) instrument, with an emphasis on methodological rigour while developing an evidence map on published recommendations for the management of HIE.
MethodsStudy design: scoping review. The study was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR).8
The protocol was recorded in the of the Open Science Framework13 registries (https://osf.io/agby9).
Inclusion criteria: CPGs on HIE in newborns; free-access publication; published in Spanish; publication date between 2013 and 2022, considering both national and international CPGs and original as well as adapted CPGs (provided the application of a formal adaptation framework, such as GRADE-ADOLOPMENT, ADAPTE, Adapted ADAPTE).
Exclusion criteria: publication type other than CPG (eg, trials, reviews, opinions, commentaries etc); CPGs based on consensus/expert opinion, single-author CPGs, CPGs published before 2013, CPGs published in languages other than Spanish, CPG not publicly available on an official website or in a peer-reviewed publication.
Sources of data: We searched for CPGs for HIE in databases, search engines, clearing houses, methodological support organizations/networks and databases of paediatrics and neonatology societies (Table 1).
Sites searched for the selection of clinical practice guidelines.
| Databases | |
|---|---|
| MEDLINE (PubMed) | https://pubmed.ncbi.nlm.nih.gov/ |
| Dimensions | https://app.dimensions.ai/discover/publication |
| LILACS | https://lilacs.bvsalud.org/es/ |
| Search engines | |
| TRIP database | https://www.tripdatabase.com/ |
| Epistemonikos | https://www.epistemonikos.org/en/groups/grade_guideline |
| Clearing houses | |
| Biblioteca de Guías de Práctica Clínica (CPG Library), National Health System of Spain | https://portal.guiasalud.es/gpc/ |
| Guías de práctica clínica (CPGs)- Mexican Social Security Institute (IMSS) | http://www.imss.gob.mx/profesionales-salud/gpc |
| Guías de práctica clínica (CPGs)– Ministry of Public Health of Ecuador | https://www.salud.gob.ec/guias-de-practica-clinica/ |
| Guías de práctica clínica (CPGs)- Colombia | https://www.sispro.gov.co/observatorios/oncalidadsalud/Paginas/Linea-Tematicas.aspx |
| Guías de práctica clínica (CPGs)- Nicaragua | https://www.minsa.gob.ni/publicaciones/direccion-general-de-regulacion-sa |
| Guías de práctica clínica (CPGs)- Chile | https://diprece.minsal.cl/programas-de-salud/guias-clinicas/ |
| Guías de obstetricia y ginecología (Obstetrics and Gynaecology guidelines) | https://www.gfmer.ch/Guidelines/Neonatologia_es/Neonatologia_mt.htm |
| National Institute for Health and Care Excellence (NICE) | https://www.nice.org.uk/ |
| Institute for Clinical Systems Improvement | https://www.icsi.org/ |
| WHO guidelines | https://www.who.int/publications/who-guidelines |
| Methodological support organizations/networks | |
| Guidelines International Network (GIN) | https://g-i-n.net/ |
| Paediatrics and Neonatology Societies | |
| Sociedad Ecuatoriana de Pediatría | https://pediatriaecuador.org/ |
| Asociación Española de Pediatría | https://www.aeped.es/especialidades |
| American Academy of Pediatrics | https://www.aap.org/en/ |
| International Pediatric Association | https://www.ipa-world.org/index.php |
| Canadian Paediatric Society | https://cps.ca/en/ |
| Sociedad Iberoamericana de Neonatología | https://www.siben.net/ |
| Sociedad Española de Neonatología | https://www.seneo.es/ |
| Asociación Colombiana de Neonatología | https://www.ascon.org.co/ |
| Other | |
Search strategy: We used the natural language search terms hypoxic ischemic encephalopathy, perinatal hypoxia, perinatal asphyxia and the controlled MeSH vocabulary terms «Hypoxia-Ischemia, Brain» [MeSH], «Hypoxia, Brain» [MeSH], «Asphyxia Neonatorum» [MeSH], «Infant, Newborn» [MeSH]; and, for filtering by type of publication, we used the natural language terms Clinical practice guide, Guideline, Clinical Guideline and the controlled vocabulary terms «Practice Guidelines as Topic» [MeSH]. We used variations of these terms according to the database. Spanish language and publication date filters from 2013 to 2022 were applied wherever possible, and free-text searches were used otherwise.
Selection of evidence sources: After the search, all identified records were compiled in a tool created for the purpose, and duplicates removed. Subsequently, two independent reviewers examined the titles and abstracts to assess them according to the inclusion and exclusion criteria. Disagreements were resolved through consensus, and a third reviewer was not required for arbitration in any instance.
Process for the creation of the PRISMA scoping review flow diagram:
Two reviewers independently extracted descriptive data from the CPGs on HIE into a spreadsheet developed for the purpose (Excel), including the title, developing organization, publication year, country, language, and method used to assess the quality of evidence.
AGREE II instrument: the Appraisal of Guidelines for Research & Evaluation (AGREE) instrument was developed to assess the variability in the quality of guidelines, evaluating methodological rigor and transparency in guideline development.9
Domain 1. Scope and Purpose, referring to the overall purpose of the guideline, specific health aspects, and the target population (items 1–3).9
Domain 2. Stakeholder Involvement, referring to the extent to which the guideline has been developed by stakeholders or interested parties and represents the viewpoints of the users it is intended for (items 4–6).9
Domain 3. Rigor of Development, referring to the process used to gather and synthesize evidence, methods for formulating and updating recommendations (items 7–14).
Domain 4. Clarity of Presentation, concerning the language, structure, and format of the guideline (items 15–17).9
Domain 5. Applicability, referring to potential barriers and facilitators for implementation, strategies to enhance adoption, and implications of applying the guideline on resources (items 18–21).9
Domain 6. Editorial Independence, the purpose of which is ensuring that the formulation of recommendations is not biased by conflicts of interest (items 22–23).9
AGREE II evaluation: Two reviewers independently evaluated each CPG using the AGREE II instrument, covering all the domains. Following the recommendations of the AGREE II developers, domain scores were calculated by adding all individual item scores in the given domain and scaling the total as a percentage of the maximum possible score for that domain.9 The criterion for high quality was defined as all a score greater than 70% in all domains.
Data analysis and description: we present the results of the descriptive analysis and the AGREE II evaluations of each guideline in tables with headings based on the elements of the data extraction instrument. The statistical analysis was performed with the software package RStudio (version 2023.12.0). We calculated the mean and standard deviation of the scores for each domain.
ResultsAfter applying an effective search strategy and the PRISMA-ScR checklist, our search yielded 356 documents. After the review of the full text, we found that 3 CPGs were eligible for inclusion in the descriptive analysis and the quality assessment using the AGREE II instrument (Fig. 1).
Two of the CPGs had been published in the 2013–2017 period and one in the 2018–2022 period. Two had been developed in Latin America Latina and one in Europe. To assess the quality of the evidence and determine the strength of recommendation, two CPGs had applied the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach and one CPG had applied the ADAPTE process (Table 2).
General characteristics of the CPGs and recommendations for the management of HIE.
| Clinical practice guideline | Organization | Year | Country | Method used to assess quality of evidence | Management: therapeutic hypothermia |
|---|---|---|---|---|---|
| Guía de práctica clínica del recién nacido con asfixia perinatal (CPG for newborns with perinatal asphyxia2 | Ministry of Health and Social Welfare | 2013 | Colombia | GRADE | The use of cooling is recommended in term newborns with moderate or severe perinatal asphyxia to prevent mortality and motor impairment at 18 months post birth. |
| Guía de práctica clínica sobre encefalopatía hipóxica-isquémica perinatal en el recién nacido (GPG on perinatal HIE in the newborn) | Ministry of Health, Social Services and Equality | 2015 | Spain | GRADE | The use of cooling is recommended in newborns delivered at or after 35 weeks’ gestation with moderate or severe perinatal HIE to reduce the risk of death or severe neurodevelopmental disability at 18−24 months post birth. |
| Encefalopatía hipóxica isquémica del recién nacido (Neonatal HIE4 | Ministry of Public Health of Ecuador | 2019 | Ecuador | ADAPTE process | The use of cooling is recommended in newborns delivered at or after 35 weeks’ gestation with moderate or severe perinatal HIE to reduce the risk of death or severe neurodevelopmental disability at 18−24 months post birth. Cooling should be initiated within 6 h of birth. |
As regards the management of HIE, we found that all guidelines recommended TH for all patients with moderate or severe HIE since this intervention has been found to reduce mortality and severe disability at 18 months post birth. Concerning gestational age, two of the guidelines recommended TH in infants delivered after 35 weeks’ gestation and one guideline recommended it for management of term newborns with perinatal asphyxia (Table 2).
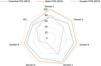
Guideline qualityFig. 2 and Table 3 present the scores for each CPG by domain.
Hypoxic-ischaemic encephalopathy CPG/AGREE II domains: scaled item scores. The chart shows that in the Spanish guideline (2015), the scores for all domains are further from the centre, which is indicative of higher quality in every evaluated domain compared to the guidelines from Colombia (2013) and Ecuador (2019).
Standardised score obtained for each of the CPGs using the AGREE II instrument.
| CPG | Scope and purpose (%) | Stakeholder involvement (%) | Rigour of development (%) | Clarity of presentation (%) | Applicability (%) | Editorial independence (%) | General recommendation | Quality |
|---|---|---|---|---|---|---|---|---|
| Colombia (2013) | 72.22% | 80.56% | 83.33% | 91.67% | 77.08% | 83.33% | Yes, with modifications | High |
| Spain (2015) | 100% | 94.44% | 92.71% | 97.22% | 89.58% | 91.67% | Yes, with modifications | High |
| Ecuador (2019) | 80.56% | 58.33% | 39.58% | 63.89% | 20.83% | 41.67% | Not recommended | Low |
Domain 1 (scope and purpose): Score of 84.26% (SD, 14.25); all guidelines (100%) had scores greater than 70%.
Domain 2 (stakeholder involvement). The score was 77.78% (SD, 18.22); two guidelines (66.66%) had scores greater than 70%.
Domain 3 (rigour of development). The score was 71.88% (SD, 28.36); two guidelines (66.66%) had scores greater than 70%.
Domain 4 (clarity of presentation). Score of 84.26% (SD, 17.86); two guidelines (66.66%) had scores greater than 70%.
Domain 5 (applicability). The score was 62.50% (SD, 36.62); two guidelines (66.66%) had scores greater than 70%.
Domain 6 (editorial independence). The score was 72.22% (SD, 26.79); two guidelines (66.66%) had scores greater than 70%.
Global evaluationOut of the three guidelines, we classified two as “recommended with modifications” and one as “not recommended”. We also classified two of the guidelines as “high quality” and one as “low quality”. Due to the limited number of guidelines included in the analysis, we did not compare the scores of the two guidelines published in the 2013–2017 period with those of the guideline published in the 2018–2022 period.
DiscussionClinical practice guidelines are statements developed with a systematic approach to guide clinicians and patients in decision-making regarding appropriate care under specific clinical circumstances.13,14 In addition, they are a valuable tool for health care administrators and policymakers, as they provide guidance on which recommendations to implement, in making health care management decisions and in developing public health policy.7 The AGREE II instrument is a tool for assessing the methodological rigour and transparency in the development of a CPG8 whose application can provide a reference that can be used to improve methodological quality, but unfortunately the quality of existing CPGs varies substantially, so that some can be contradictory or not be up to date.15
In our study, we identified 3 CPG that satisfied the eligibility criteria, of which 2 ended up classified as “high quality” and “recommended with modifications”. In a similar study, Amer et al. identified 2 CPGs on HIE whose quality they also evaluated with the AGREE II instrument, and the authors concluded that one of them was of superior methodological quality.3
In our quality evaluation, the highest scores corresponded to the “scope and purpose” and “clarity of presentation” domains. Overall, all 3 CPGs included clear information regarding both domains; when it came to “scope and purpose”, all addressed most of the AGREE II criteria, describing not only the purposes and the target population but also the health care aspects covered by the guideline, such as the diagnosis and management of HIE, which are important as they are directly related to the potential impact that the guideline can have on the population16; when it came to the “clarity of presentation” domain, the recommendations were specific and easy to identify, making them particularly useful for the purpose of decision-making in clinical practice.
Conversely, the domain with the lowest scores was the “applicability” domain, which was consistent with the findings of two systematic reviews that assessed methodological quality in paediatric CPGs.3,17 These low scores may be related to the development of guidelines in the abstract without consideration of their translation into implementation processes18; in addition, the assessment of applicability also considers aspects concerning implementation strategies and what they would entail in terms of resources,8 something that can be challenging for CPGs to address comprehensively, as implementation often involves health policy decisions that are usually not in the power of the authors, and even official institutions that endorse guidelines do not always commit to their implementation at their national or regional scope of authority.
When it came to stakeholder involvement, all three guidelines were developed by multidisciplinary teams related to HIE, and we ought to highlight that the authors of the guidelines developed in Spain19 and in Colombia20 engaged in qualitative research to take into account the values and preferences of parents of neonates with HIE in the development of recommendations. The integration of the values and preferences of the target population is important, as it promotes patient-centred care and ensures the acceptability of the recommendations. This can be achieved by including patients in the expert groups, performing reviews of the literature on the values and preferences of patients or through external reviews of the CPG document by patients, among other possibilites.21
The score in domain 3, which refers to the rigour of development, is considered the best predictor of the overall quality of the guideline, as a high score in this domain is indicative of minimal bias and development of recommendations based on scientific evidence.22 In our study, the mean score in this domain was 71.88%, and two of the three guidelines had scores greater than 70%.
In addition, our study found that the CPG with the highest methodological rigour was developed in Spain, while the CPG with the lowest rigour was developed in Ecuador, findings that were consistent with those of the systematic review conducted by Amer et al., who classified as superior the guideline developed by organizations in high-income countries.3 In middle- to low-income countries, the development of CPGs has slowly and gradually adapted to the standards of high-income countries.21 Thus, Canelo-Aybar et al., who evaluated the methodological quality of the 17 CPGs developed by the Ministry of Health of Peru, concluded that the methodological quality was low and did not recommend their use23; similarly, Esandi et al. evaluated 101 CPGs developed in Argentina, whose recommendations scored very low in nearly every domain.24 These results could be attributed to the fact that in middle- to low-income countries, certain factors, like the limited availability of resources, preclude thorough investigation and therefore affect the methodological rigour; the lack of staff with the skills required for guideline development or the barriers posed by the health care system itself hindering the implementation of guidelines are among the factors that could be contributing to the low methodological quality of the CPGs developed in these countries.
In our study, we found that every CPG recommended the use of TH for management of HIE, in agreement with the systematic review by Amer et al., in which all evaluated guidelines supported its use.3 At present, TH is the standard of care for newborns with moderate to severe HIE,25–29 as multiple trials have reported improved neurodevelopmental outcomes at 18 months post birth with its use and it is the only effective and safe neuroprotection strategy currently available for moderate to severe HIE.28,30,31 Consequently, we support the recommendation of including TH as part of the management of newborns with HIE in current CPGs. However, studies conducted in middle- to low-income countries have reported that TH has a limited impact in reducing mortality in newborns with HIE and one even concluded that it should not be recommended for management of HIE in middle- to low-income countries,9,10 so additional studies of larger scope are required to better understand the usefulness of TH in resource-limited settings. It is essential to keep in mind that CPGs in such settings, for instance, in Ecuador or Colombia, may require adjustments based on the local needs and accessibility of health care resources.
Among the limitations of our study are the small number of CPGs on HIE available, which could have hindered a comprehensive understanding with the proposed approach, and the exclusion of publications in languages other than Spanish, which may have left out relevant CPGs for HIE. In addition, while the assessment using the AGREE II tool was carried out by two appraisers, the developers of the tool recommend its application by four appraisers, which may have affected the reliability of the evaluation. The chief strength of the study is the effort made to find the greatest possible number of guidelines meeting the inclusion criteria by searching not only in databases but also in several registries and repositories. It is also pioneering in that, to our knowledge, no previous study has analysed the quality of CPGs for the management of HIE published in Spanish using the AGREE II checklist.
ConclusionOur study resulted in the classification of two of the analysed guidelines as high quality resources. However, we identified limitations in their applicability in addition to differences in methodological rigour between guidelines developed in high-income versus low- or middle-income countries. It is important that the development of guidelines improve their local adaptation by considering the target health system, although this may prove challenging since this often requires public policy measures. Thus, CPGs are also valuable tools for health care administrators and public health policymakers. In addition, taking into account the values and preferences of patients promotes patient-centred care.
Considerable efforts need to be invested to make available high-quality guidelines for the management of HIE in newborns and adapted to local conditions.
FundingThis research project did not receive specific financial support from funding agencies in the public, private or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.