Growth hormone (GH) and insulin-like growth factor-1 (IGF-1) have modulatory effects on bowel function and its microbiota. Our aim was to investigate whether low levels of GH and IGF-1 in patients with GH deficiency are associated with changes in gut physiology/integrity as well as in the composition of the gut microbiota.
Materials and methodsWe conducted a case-control study in 21 patients with GH deficiency, at baseline and after 6 months of GH treatment, and in 20 healthy controls. We analysed changes in anthropometric and laboratory characteristics and bacterial translocation and studied the composition of the microbiome by means of massive 16S rRNA gene sequencing.
ResultsGrowth hormone deficiency was accompanied by a significant increase in serum levels of sCD14, a marker of bacterial translocation (P < .01). This increase was reversed by GH treatment. We did not find any differences in the composition or α- or β-diversity of the gut microbiota after treatment or between cases and controls.
ConclusionsOur work is the first to demonstrate that the presence of GH deficiency is not associated with differences in gut microbiota composition in comparison with healthy controls, and changes in microbiota composition are also not found after 6 months of treatment. However, GH deficiency and low IGF-1 levels were associated with an increase in bacterial translocation, which had reversed after treatment.
La hormona de crecimiento (GH) y el factor de crecimiento similar a la insulina tipo 1 (IGF-1) tienen efecto modulador sobre la funcionalidad intestinal y la microbiota. Nuestro objetivo fue investigar si pacientes con déficit de GH y por lo tanto niveles bajos de GH e IGF-1 se asocian con cambios en la fisiología/integridad intestinal así como en la composición de la microbiota intestinal.
Material y métodosSe realizó un estudio de casos y controles en 21 pacientes con déficit de GH previo al inicio y tras 6 meses de tratamiento con GH y en 20 controles sanos. Se estudiaron los cambios antropométricos, analíticos, de translocación bacteriana y también se determinó la composición del microbioma mediante secuenciación masiva del gen del ARNr 16S.
ResultadosEl déficit de GH se acompañó de un incremento significativo en los niveles séricos de sCD14, un marcador de translocación bacteriana (p < 0.01). Dicho incremento fue revertido por el tratamiento con GH. No se observaron diferencias en la composición, ni en α o la β-diversidad de la microbiota intestinal entre los casos y los controles, ni tras recibir tratamiento.
ConclusionesNuestro trabajo demuestra por primera vez que un déficit de GH no se acompaña de cambios en la composición de la microbiota intestinal en comparación con controles sanos, ni tampoco se ve modificada tras 6 meses de tratamiento. Sin embargo, este déficit de GH y niveles bajos de IGF-1 si se asoció con un incremento en la traslocación bacteriana, que se vio revertido tras el tratamiento.
Microbiota is defined as the community of microorganisms living in a specific environment. Generally, commensal microorganisms live in every epithelial surface of the body. The ratio of bacteria to human cells in the body is 1.3 to 1, and the total weight of these bacteria ranges from 0.2 to 0.4 kg. The microbiota could be considered the last organ of the human body, so that the latter could be considered a metaorganism or superorganism encompassing its (own) cells and all the microbes inhabiting the body.1 The term “microbiota” is used nearly exclusively to refer to the bacterial component and its chiefly composed by 4 bacterial phyla: Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria.2
The establishment of the microbiota is a lifelong dynamic process.3–5 It is influenced by factors such as the type of delivery, perinatal antimicrobial exposure or the infant feeding modality.6 Later in life, the composition and functioning of the microbiota is not set and can be affected by diet, antimicrobial use and other events (puberty, pregnancy, menopause).7,8
Complex microbial ecosystems can be found in different regions of the body. The most populous and complex is the gut microbiota, especially in the ileum and colon, where the bacterial component has adapted to life in the intestinal lumen. These communities have a symbiotic and mutualistic interaction with human eukaryotic cells and are necessary for the correct functioning of the human organism. Changes in the composition or functioning of the microbiota (dysbiosis) have been associated with various diseases.9,10
Among the different functions performed by the microbiota, we ought to highlight its role in growth.11 Indeed, several studies have demonstrated that germ-free mice, lacking a gut microbiota, have decreased levels of insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein 3 (IGFBP-3) and exhibit growth restriction, with a decrease in linear growth and weight gain, which suggests that mammals need the gut microbiota to achieve optimal growth.12–14 In this regard, clinical studies in animal models have demonstrated that physiological levels of growth hormone (GH) maintain the integrity of the gut and improve gastrointestinal functioning. This effect of GH happens synergistically with IGF-1, as exemplified in bone growth, metabolism, and intestinal homeostasis,15–18 indicating a potential relationship between GH/IGF-1 and the gut microbiota.
Somatostatin is secreted by the hypothalamus and inhibits GH in addition to preventing gastric acid secretion and slowing gut motility. Studies in mice models of congenital diarrhoeal disorders have found that these mice had increased levels of somatostatin and decreased gut hormone levels in addition to dysbiosis of the gut microbiota, which again points at the potential association between the gut microbiota and the GH/IGF-1 axis, although further research on the subject is still required.15,19
The prevalence of GH deficiency in the paediatric population is of at least 1 in 3480 children, and the disorder can be isolated or associated to other pituitary hormone deficiencies. Clinically, it is characterised by proportionate postnatal growth. Affected individuals exhibit a reduced response to stimulation tests and decreased IGF-1 and IGFBP-3 levels. The indicated treatment is replacement with recombinant human GH.20 In this context, and taking into account the laboratory characteristics of these patients, with decreased levels of GH and IGF-1, we conducted a study with the aim of determining whether these abnormalities were associated with changes in intestinal physiology/integrity and the composition of the gut microbiota.
Material and methodsStudy designSingle centre case-control study including a total of 42 participants aged 4 to 14 years (21 cases and 20 controls). Cases underwent longitudinal follow-up to compare the gut microbiota and assess bacterial translocation before and after 6 months of treatment with GH.
We recruited cases from the paediatric endocrinology clinic of a tertiary care hospital (May 2020–June 2022) right before the start of GH replacement therapy. The sample included those patients who met the diagnostic criteria for GH deficiency: clinical growth retardation and decreased response in 2 GH stimulus tests (GH < 7 ng/mL).20
The controls were patients matched for age and sex. We included children who were scheduled to undergo a blood draw in the preoperative assessment before undergoing trauma, urological or ear, nose, throat surgery.
We excluded patients with infectious or autoimmune disease, malabsorption syndrome, obesity or short stature in absence of GH deficiency. Patients who had been treated with antibiotics, probiotics or immunosuppressants in the past 3 months were also excluded.21
The study was approved by the regional Ethics Committee for Research with Medicines (file no.PI-399). We obtained informed consent from the parents or legal guardians of the patients.
Biochemical variablesWe measured levels of glucose, total cholesterol, high-density lipoprotein (HDL), low density lipoprotein (LDL) and triglycerides (c702 module, Cobas 8000 analyser series, Roche Diagnostics), insulin by electrochemiluminescence (e802 unit, Cobas 8000 analyser series, Roche Diagnostics), glycated haemoglobin (HbA1c) (Bio-Rad D100 analyser) and IGF-1 and IGFBP-3 by chemiluminescence (Immulite 2000, Siemens).
Bacterial translocationWe measured the serum levels of soluble cluster soluble of differentiation 14 (sCD14) and lipopolysaccharide binding protein (LBP) by means of Luminex technology, a type of immunoassay that can obtain accurate measurements in multiple simultaneous assays in a single sample. The Luminex xMAP technology is a bead-based multiplexed immunoassay system allowed the detection of up to 100 targets simultaneously, similar to what had been done in previous studies by our group.22,23
DNA extraction, 16S rRNA sequencing and bioinformatic analysisParticipants collected faecal samples in a sterile container following the printed instructions provided by the research team, and the samples were aliquoted and stored at −80 °C for subsequent analysis. Then, the faecal samples were thawed and faecal DNA extracted using the Real Microbiome Fecal DNA kit (Durviz, Valencia, Spain) following the directions of the manufacturer. The purity, concentration and quality of the obtained DNA were assessed with a NanoDrop 1000 spectrophotometer (Thermo Scientific, USA), a Qubit 3.0 fluorometer (Thermo Fisher Scientific, MA, USA) and a fragment analyser (Agilent, USA).
The samples were subjected to amplification of the V3-V4 hypervariable regions of the bacterial 16S rRNA gene. Amplicon sequencing was carried out with an Illumina system (MiSeq, 2 × 300 bp, paired-end) in the genomics and bioinformatics platform of the biomedical research centre. The first step thereafter was to assess the quality of the reads by means of the FastQC programme.24 Then we used the Qiime2 plugin25 for the bioinformatic analysis. We started by denoising demultiplexed (by sample, based on barcoding) raw read files using the software DADA2.26 This step included trimming of adaptor sequences and primers, filtering of low-quality reads, sequence dereplication to reduce repetition, merging of paired-end reads, the reconstruction of amplicon sequence variants (ASVs) and the removal of chimeras. Secondly, we used the SILVA database27 trained with the primers used for amplification of the V3-V4 regions for taxonomic assignment. The next step was analysing α and β diversity: α diversity is a measure of the diversity of species in a given sample, while β diversity is a measures of the similarities between microbial communities in different samples, facilitating the identification of differences between them. We assessed α diversity with the following metrics: observed features, Chao1 index and Fisher α, indicators of species richness, the Pielou index of species evenness and the Shannon index and Simpson index of species diversity (richness + evenness). We assessed β-diversity by means of permutational ANOVA (PERMANOVA) (999 permutations) with Bray Curtis distances and visualised the results by means of principal coordinate analysis (PCoA) using the R Studio software (version 1.4.1105). Last of all, we analysed differential abundance with the ANCOM methodology28 at the phylum, order and genus levels. This methodology is based on the underlying data structure and is widely used to compare microbiome composition in 2 or more populations without assumptions regarding the distribution of the population.
Statistical analysisThe statistical analysis was carried out with the software SPSS version 22 (SPSS Inc. Chicago, IL, USA) and Prism 8 (GraphPad Prism, La Jolla, California, USA), defining statistical significance as a P value of less than 0.05.
We used the Shapiro-Wilk test to assess the normality of distributions.
To compare 2 groups, we used the independent samples t test or the Mann-Whitney U test based on whether the data followed a normal distribution. In the case of longitudinal analyses, we compared the data by means of the paired sample t test or the Wilcoxon test based on whether they followed a normal distribution.
To compare qualitative variables, we used the χ2 test or, if the conditions required for it were not met, the Fisher exact test. We compared the three groups by means of analysis of variance (ANOVA) followed by the post hoc Tukey test.
ResultsDemographic, biochemical and metabolic characteristics of cases compared to controls and to cases after treatment with growth hormoneThe mean age was 1 year younger in controls compared to cases (8.08 vs 9.11 years). As regards pubertal development, only 10% of controls had undergone onset of puberty compared to 28.6% of cases, although the difference was not statistically significant. None of the cases had GH deficiency secondary to another disease or GH deficiency combined with other pituitary hormone deficiencies.
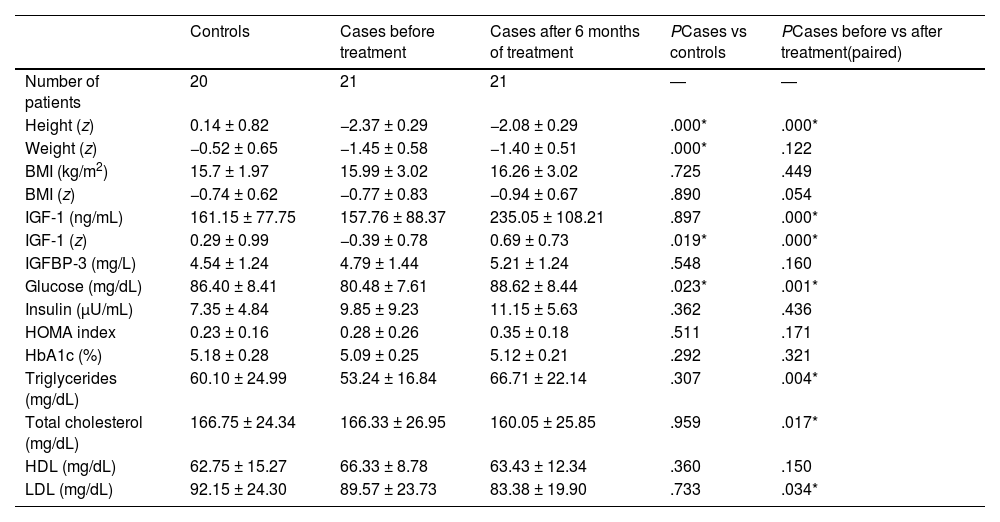
Table 1 compares the control group, the case group at baseline and the case group at 6 months of treatment. As expected, we found a lower height in cases and improvement in height after treatment with GH (P < .01). We did not find changes in weight, although there was a reduction in the body mass index (BMI) z score with a P value that neared, but did not reach, statistical significance (P = .054).
Comparison of controls and patients with growth hormone deficiency at baseline and after 6 months of treatment.
| Controls | Cases before treatment | Cases after 6 months of treatment | PCases vs controls | PCases before vs after treatment(paired) | |
|---|---|---|---|---|---|
| Number of patients | 20 | 21 | 21 | ― | ― |
| Height (z) | 0.14 ± 0.82 | −2.37 ± 0.29 | −2.08 ± 0.29 | .000* | .000* |
| Weight (z) | −0.52 ± 0.65 | −1.45 ± 0.58 | −1.40 ± 0.51 | .000* | .122 |
| BMI (kg/m2) | 15.7 ± 1.97 | 15.99 ± 3.02 | 16.26 ± 3.02 | .725 | .449 |
| BMI (z) | −0.74 ± 0.62 | −0.77 ± 0.83 | −0.94 ± 0.67 | .890 | .054 |
| IGF-1 (ng/mL) | 161.15 ± 77.75 | 157.76 ± 88.37 | 235.05 ± 108.21 | .897 | .000* |
| IGF-1 (z) | 0.29 ± 0.99 | −0.39 ± 0.78 | 0.69 ± 0.73 | .019* | .000* |
| IGFBP-3 (mg/L) | 4.54 ± 1.24 | 4.79 ± 1.44 | 5.21 ± 1.24 | .548 | .160 |
| Glucose (mg/dL) | 86.40 ± 8.41 | 80.48 ± 7.61 | 88.62 ± 8.44 | .023* | .001* |
| Insulin (μU/mL) | 7.35 ± 4.84 | 9.85 ± 9.23 | 11.15 ± 5.63 | .362 | .436 |
| HOMA index | 0.23 ± 0.16 | 0.28 ± 0.26 | 0.35 ± 0.18 | .511 | .171 |
| HbA1c (%) | 5.18 ± 0.28 | 5.09 ± 0.25 | 5.12 ± 0.21 | .292 | .321 |
| Triglycerides (mg/dL) | 60.10 ± 24.99 | 53.24 ± 16.84 | 66.71 ± 22.14 | .307 | .004* |
| Total cholesterol (mg/dL) | 166.75 ± 24.34 | 166.33 ± 26.95 | 160.05 ± 25.85 | .959 | .017* |
| HDL (mg/dL) | 62.75 ± 15.27 | 66.33 ± 8.78 | 63.43 ± 12.34 | .360 | .150 |
| LDL (mg/dL) | 92.15 ± 24.30 | 89.57 ± 23.73 | 83.38 ± 19.90 | .733 | .034* |
Quantitative variables expressed as mean ± standard deviation. The P value refers to the comparison of 2 groups. Asterisks (*) indicate statistically significant results (P < .05).
BMI, body mass index; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; IGFBP-3, insulin-like growth factor binding protein 3; IGF-1, insulin-like growth factor 1; LDL, low-density lipoprotein.
We found a statistically significant difference in IGF-1 levels between cases and controls and an increase in serum IGF-1 levels in cases after initiating treatment, as expected. We also found an increasing trend in IGFBP-3 values after treatment, although the difference was not statistically significant.
After initiating treatment with GH, the only significant change observed in glucose metabolism was the serum glucose level (P < .01), with no changes in insulin or HbA1c levels or in the HOMA insulin resistance index.
As regards lipid metabolism, we found a significant reduction in total cholesterol and LDL levels after treatment with GH (P < .01 and P < .05, respectively). On the other hand, there was also an increase in serum triglyceride levels (P < .01) after treatment.
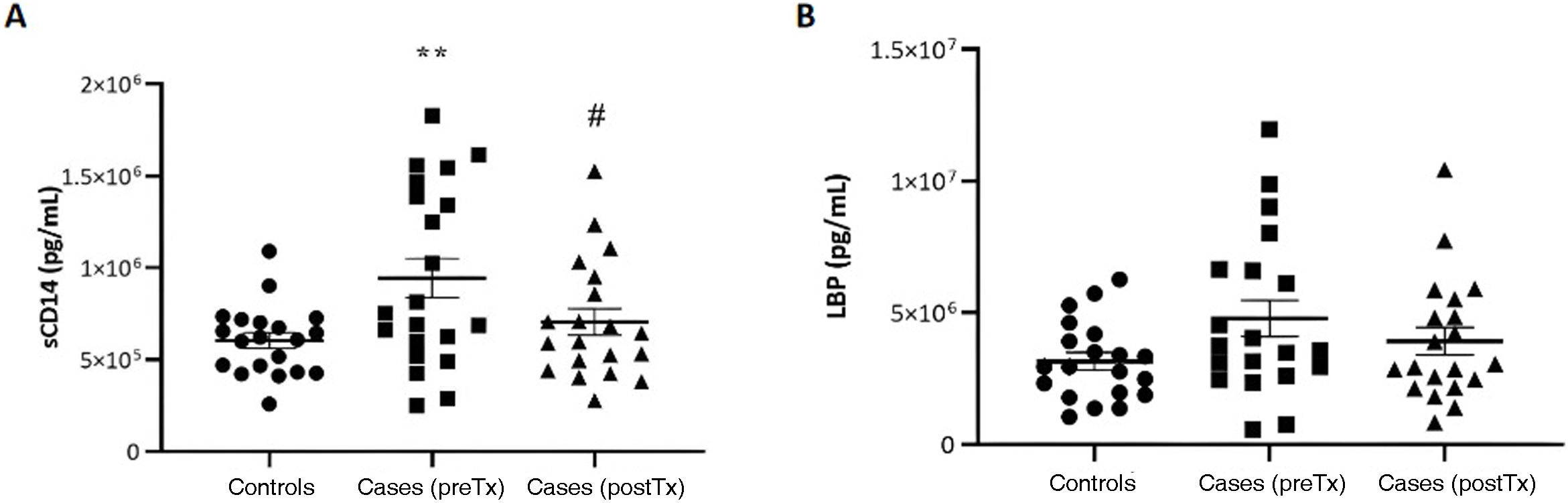
Bacterial translocationFig. 1 presents the results of the analysis of bacterial translocation.
We found an increase in sCD14 levels compared to controls (P < .01) (Fig. 1A). Treatment with GH achieved recovery of these levels (P < .05). The pattern was very similar when we analysed the levels of LBP, although in this case the differences were not statistically significant (Fig. 1B).
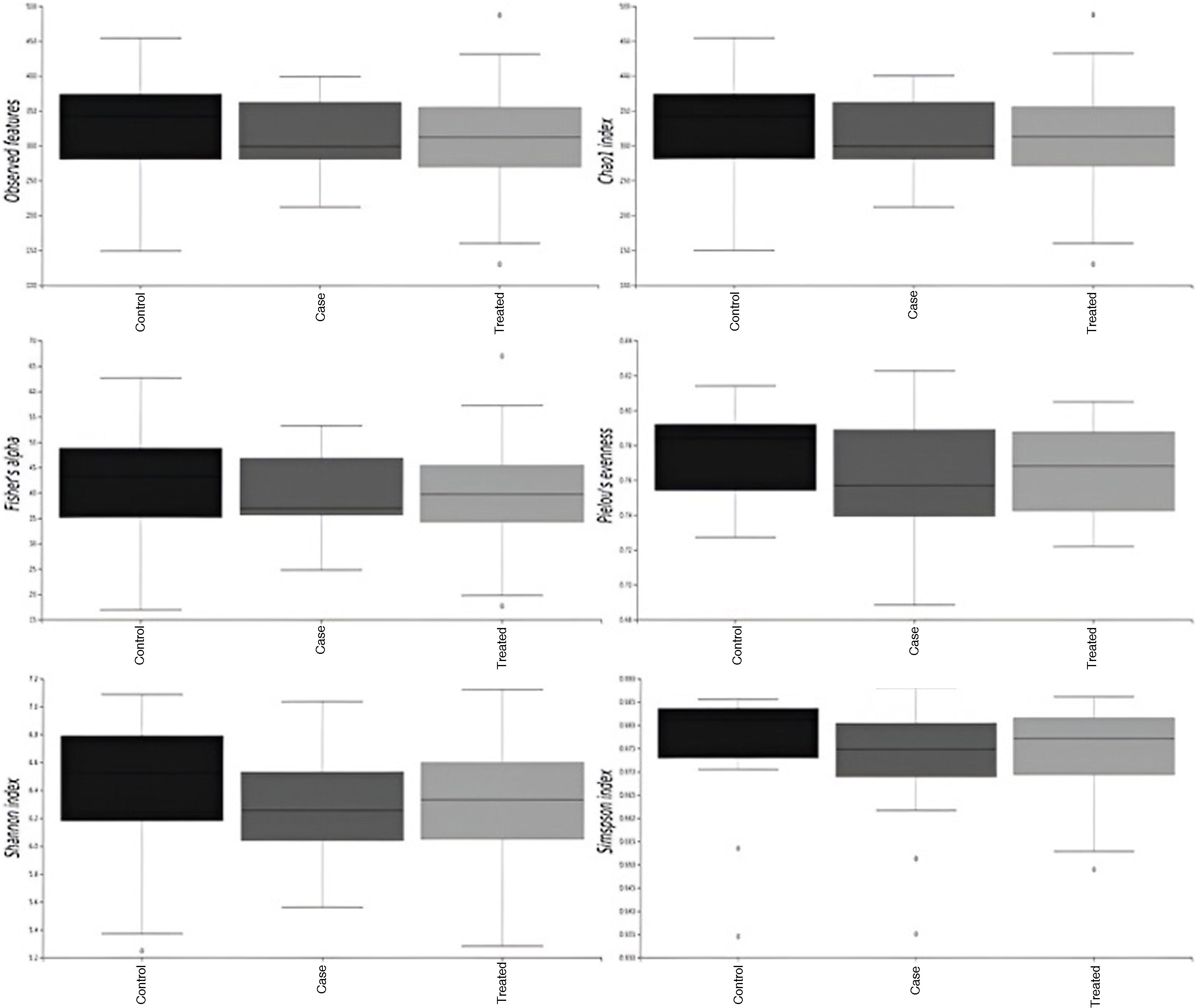
Alpha diversity of the gut bacteriomeWe compared cases and controls before and after treatment. We did not find statistically significant differences between the groups in any of the analysed indices, although in the case group values tended to be lower and exhibited an increasing trend after 6 months of GH replacement therapy (Fig. 2).
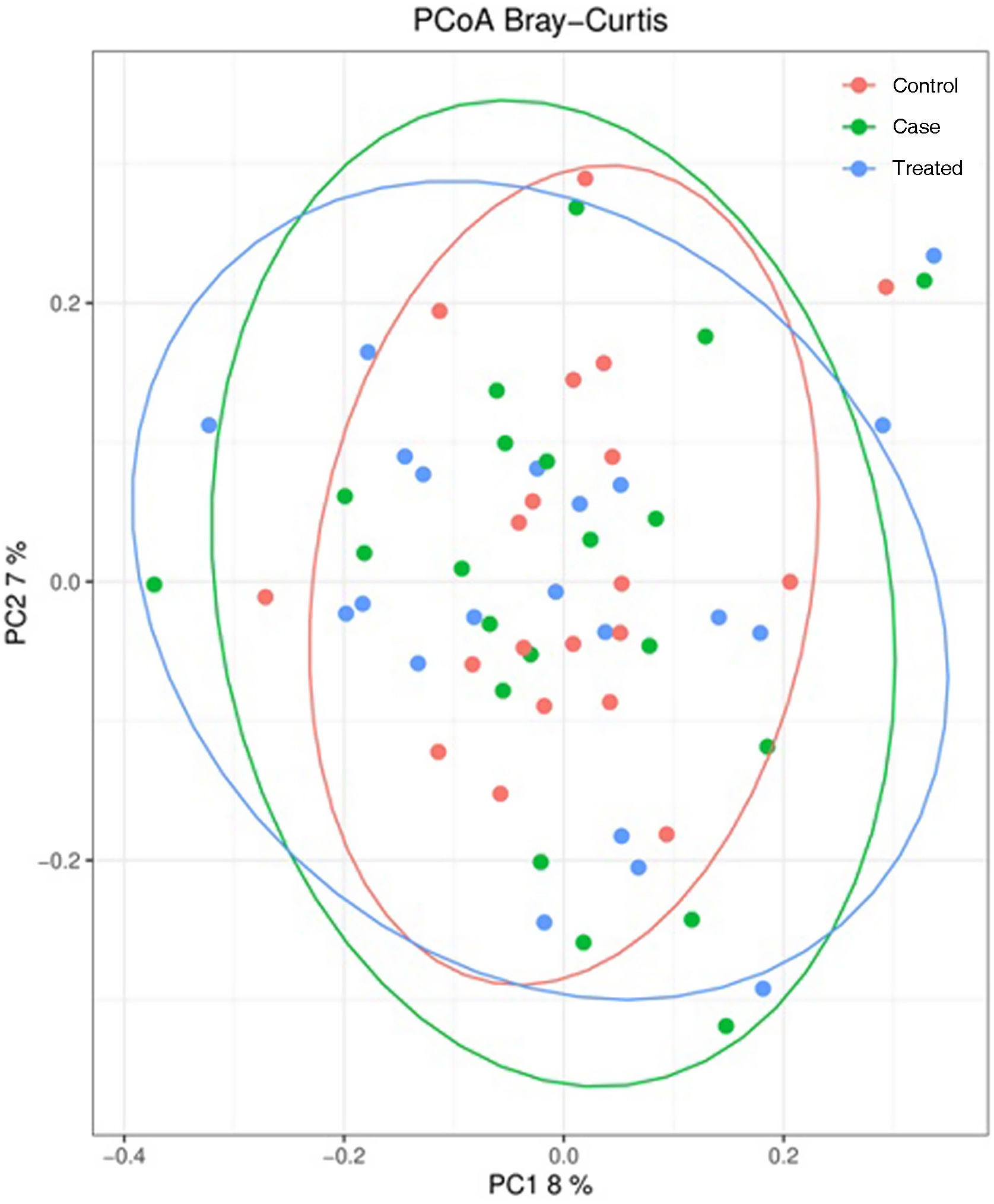
Beta-diversity of the gut bacteriomeFig. 3 presents the principal coordinate analysis of the comparison of bacterial communities in the different groups under study. We did not find statistically significant differences between groups.
Principal component analysis of bacterial communities in stool samples of participants (15% of total variance [component 1 = 8% and component 2 = 7%]). Results are presented according to the 2 principal components. Each dot represents a sample: red dots represent controls, green dots represent cases and blue dots cases after treatment. The grouping of samples is represented through the 95% confidence ellipsoid.
PC, principal component; PCoA, principal component analysis.
As regards relative abundance, we did not find statistically significant differences in bacterial taxa between the 3 groups under study, overall or at the phylum, genus or species level.
DiscussionOur study is the first to describe the composition of the gut microbiota in children with GH deficiency compared to healthy controls as well as changes in the composition of the gut microbiota after 6 months of GH replacement therapy.
Glucose levels had increased significantly after treatment with GH. This hormone increases insulin sensitivity through the improvement of body composition on account of an increase in lean mass and a reduction in fat mass. However, it also stimulates glycogenolysis, gluconeogenesis and lipolysis, thereby increasing serum glucose levels. This has been previously studied in patients with GH deficiency in whom treatment was discontinued, who exhibited a decrease in glucose levels that then increased again once treatment resumed.29 In fact, a review of studies on the metabolic changes caused by treatment with GH found an initial worsening in glucose metabolism due to a reduction of insulin sensitivity followed by improvement over the long term due to the beneficial effects of treatment on body composition, which counteract the insulin-antagonist effect of GH, resulting in an overall increase in insulin sensitivity.30
Our study also found changes in the lipid profile after 6 months of treatment with GH, as children exhibited a significant decrease in total cholesterol and LDL levels, in agreement with the previous literature,29 since GH promotes the use of fat as a source of energy and therefore a reduction in adiposity and an increase in lean mass. On the other hand, we did not observe changes in HDL levels, which was also consistent with other studies, a finding that may be attributed, at least in part, to the short duration of follow-up after treatment initiation.31,32
The increase in bacterial translocation observed in children with GH deficiency is of great interest, as it suggests an increase in gut permeability and therefore in the passage of microorganisms or parts thereof to the bloodstream, which in turn can trigger an inflammatory cascade in different tissues, with a negative impact on the health of the individual, since increased bacterial translocation is associated with chronic inflammation and increased cardiovascular risk.10 This increase in bacterial translocation could be related to the low levels of IGF-1 observed in these children, as demonstrated in a previous study.33
Previous studies have also found that the IGF-1 receptor (IGF1R), through which IGF-1 carries out its functions, can modulate gut barrier function.34 In this regard, our study showed that treatment with GH was associated with a significant increase in IGF-1 and IGFBP-3, similar to the increases reported in previous studies,35 accompanied by a decrease in bacterial translocation,18,36 which highlights the bidirectional association between IGF-1 and gut/microbiota function.13
However, these changes in bacterial translocation were not associated with changes in the composition of the microbiota. In fact, our study did not evince differences in the composition of the bacteriome between healthy children (controls) and children with GH deficiency, despite the detection of statistically significant differences in IGF-1 levels between the two groups. Treatment with GH increased these levels, as expected, but this increase was not associated with changes in the gut microbiota in terms of alpha or beta diversity or differential abundance. A possible explanation is that the increase in IGF-1 levels is not large enough to result in changes at the level of the microbiota or that a longer duration of follow-up is required for these changes to be detectable. In fact, studies in mice have found that the microbiota is more immature in those without GH, with lesser diversity at the genus level and reduced levels of bacteria of the Proteobacteria, Campylobacteria and Actinobacteria phyla.12,14,37–39 We may have failed to detect differences in our study due to the participation of patients who did not have total GH deficiency (GH < 3 ng/mL after 2 stimulation tests),20 as most participants had partial GH deficiency. In fact, a study that analysed the characteristics of the microbiota in patients with GH-secreting pituitary adenoma40 found changes in the microbiota, with a decreased microbial diversity in patients with excess GH and statistically significant changes in both α and β diversity. In fact, the authors reported a change by more than 400 ng/mL in the IGF-1 level of patients when comparing preoperative and postoperative levels. In our study, the changes observed in patients after treatment, despite being statistically significant, did not reach 100 ng/mL in magnitude, which could also explain, at least in part, the absence of changes in composition in the microbiota of our patients.
The main limitations of our study are the small size of each of the groups in the sample, the inclusion of patients with partial GH deficiency and the short follow-up duration of 6 months. A larger sample would allow a stratified analysis by age or pubertal stage. Further studies are required in larger samples and with a longer duration of treatment and longer duration of follow-up to assess changes in patients after discontinuation of GH treatment.
ConclusionOur study is the first to demonstrate that GH deficiency (growth retardation and decreased response [<7 ng/mL] in 2 GH stimulation tests) in children is not associated with changes in the gut microbiota compared to the microbiota of healthy children and that the gut microbiota of children treated with GH for 6 months also does change compared to baseline. However, growth retardation and decreased levels of IGF-1 and GH were associated with increased bacterial translocation, which was reversed with treatment, underscoring the beneficial effects of GH and IGF-1 not just on growth but also on intestinal integrity and, by extension, of all the axes associated with it: gut-brain axis, gut-liver axis, etc. However, more studies are required (with long durations of treatment or in patients with more severe GH deficiency) to understand in detail the association between GH/IGF-1 and the gut and its impact on child health, both in the short and the long term.
FundingThis study was funded by the Instituto de Estudios Riojanos (IER) through grants for scientific research concerning La Rioja.
Conflicts of interestThe authors have no conflicts of interest to declare.






![Principal component analysis of bacterial communities in stool samples of participants (15% of total variance [component 1 = 8% and component 2 = 7%]). Results are presented according to the 2 principal components. Each dot represents a sample: red dots represent controls, green dots represent cases and blue dots cases after treatment. The grouping of samples is represented through the 95% confidence ellipsoid. PC, principal component; PCoA, principal component analysis. Principal component analysis of bacterial communities in stool samples of participants (15% of total variance [component 1 = 8% and component 2 = 7%]). Results are presented according to the 2 principal components. Each dot represents a sample: red dots represent controls, green dots represent cases and blue dots cases after treatment. The grouping of samples is represented through the 95% confidence ellipsoid. PC, principal component; PCoA, principal component analysis.](https://static.elsevier.es/multimedia/23412879/0000010000000006/v2_202407150516/S2341287924001431/v2_202407150516/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w95erwEulN6Tmh1xJpRhO+VE=)


