Many antiviral agents, such as hydroxychloroquine, have been used to treat COVID-19, without being broadly accepted. QTc prolongation is a worrisome adverse effect, scarcely studied in pediatrics.
Patients and methodsPaediatric patients affected from COVID-19 who received antivirals were matched (1:2) with controls not infected nor exposed. Electrocardiograms were prospectively analyzed at baseline, during the first 72 h of treatment and after 72 h.
ResultsEleven (22.9%) out of 48 patients admitted due to COVID-19 (March–July 2020) received antiviral therapy. All had underlying diseases: congenital heart disease (4/11; 36.4%) and immunosuppression (3/11; 27.3%) stand out. 5/11 (45.5%) received treatment at baseline with a potential effect on QTc. There where no differences observed in the baseline QTc between cases and controls: 414.8 ms (49.2) vs 416.5 ms (29.4), (P = .716). Baseline long QT was observed in 2/11 cases and 2/22. Among cases, 10/11 (90.9%) received hydroxychloroquine, mainly associated with azithromycin (8/11; 72.7%), 3 received lopinavir/ritonavir and one remdesivir. The median increase in QTc after 72 h under treatment was 28.9 ms [IQR 48.7] (P = .062). 4/11 (36.4%) patients had a long QTc at 72 h, resulting in 3 patients ≥500 ms; treatment was stopped in one (QTc 510 ms) but ventricular arrhythmias were not documented.
ConclusionsThe use of antivirals caused an increase on the QTc interval after 72 h of treatment, being the QTc long in 36.3% of the patients, although no arrhythmic events were observed. The use of hydroxychloroquine and antivirals requires active QTc monitoring and it is recommended to discontinue treatment if QTc > 500 ms.
Muchos antivirales, como hidroxicloroquina, se han utilizado para el tratamiento de COVID-19. La prolongación del QTc es un efecto adverso preocupante, escasamente estudiado en pediatría.
Pacientes y métodosLos pacientes pediátricos con COVID-19 que recibieron tratamiento antiviral se emparejaron (1:2) con controles no infectados ni expuestos al tratamiento. Se analizaron prospectivamente los electrocardiogramas basal, en las primeras 72 horas de tratamiento y posterior a 72 horas.
ResultadosOnce (22,9%) de 48 pacientes pediátricos ingresados por COVID-19 (Marzo-Julio 2020) recibieron terapia antiviral. Todos presentaban patologías de base; destacando cardiopatías (4/11; 36,4%) e inmunosupresión (3/11; 27,3%); 5/11 (45,5%) recibían tratamiento de base con potencial efecto sobre el QTc. No hubo diferencias en el QTc basal entre casos y controles: 414,8 ms (49,2) vs 416,5 ms (29,4) (P = ,716). Se observó QTc prolongado basal en 2/11 casos y 2/22 controles. De los casos, 10/11 (90,9%) recibieron hidroxicloroquina, principalmente asociada a azitromicina (8/11; 72,7%); tres recibieron lopinavir/ritonavir, uno remdesivir. La mediana de incremento del QTc tras 72 horas fue de 28,9 ms [RIC 48,7] (P = ,062); 4/11 (36,4%) presentaron un QTc largo, de los cuáles en tres ≥500 ms. En uno se paró el tratamiento (QTc 510 ms) pero no se documentaron arritmias ventriculares.
ConclusionesEl uso de fármacos antivirales causó un incremento del QTc tras 72 horas de tratamiento, considerándose un QTc largo en el 36,4% de los pacientes, aunque no se objetivaron eventos arrítmicos. El uso de hidroxicloroquina y antivirales requiere monitorización activa del QTc y se recomienda suspender el tratamiento si el QTc >500 ms.
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19) was declared a pandemic and global health emergency by the World Health Organization on January 30, 20201 and continues to be the main concern of health care systems worldwide, with more than 100 million confirmed cases and 2 500 000 deaths recorded to date.1,2 In Spain, there have been more than 3 000 000 confirmed cases of COVID-19 and more than 70 000 related deaths to date.1,3
In the early literature on COVID-19, severe cases in the paediatric population were only reported exceptionally, but as time went by, the number of cases described in this age group has grown,4–6 although the severity of the disease has continued to be far lower compared to the adult population. In Spain, only 0.8% of the total cases were in individuals under 18 years.4
At present, there are no authorised or universally accepted therapies for aetiological treatment of COVID-19. While numerous agents have been investigated and multiple studies published, there is still no widely accepted antiviral treatment.2,7,8
The first published studies that presented limited data on the in vitro suppression of SARS-CoV-2 activity led to the use of drugs in the aminoquinoline group: chloroquine and hydroxychloroquine, previously used for treatment of Ebola, H7N9 influenza and severe acute respiratory syndrome (SARS) associated with coronavirus infection.2,7 The treatment regimens varied between countries and hospitals, in the clinical indication for their used and in published studies.9,10 Current studies with more robust methods have not been able to replicate past results, and these drugs carry a risk of harm to the patient, as their use has been associated with several potential adverse effects, most importantly those involving the heart.2,7,11,12
Among the adverse events documented in association with the use of hydroxychloroquine, QT interval prolongation and severe ventricular arrhythmias are among the most alarming,13,14 especially when combined with other drugs with similar potential adverse effects, such as azithromycin.11,15
The evidence on electrocardiographic abnormalities in paediatric patients with COVID-19 is scarce.16 There is also a dearth of data on the use of hydroxychloroquine and other antiviral drugs in large series of paediatric patients outside the context of the COVID-19 pandemic. The association between hydroxychloroquine and heart conduction disorders, well established in adults, continues to be unpredictable in paediatric patients, and only one study in paediatric patients with systemic lupus erythematosus has attempted, unsuccessfully, to establish this association.17
The aim of our study was to assess the degree of QT interval prolongation in paediatric patients with COVID-19 managed in our hospital in relation to the administration of hydroxychloroquine and antiviral drugs recommended for treatment of COVID-19: lopinavir/ritonavir, remdesivir and azithromycin.
Sample and methodsWe selected patients from the total population of paediatric patients admitted to the Hospital Universitario Vall d’Hebron (Barcelona, Spain) due to COVID-19 confirmed by polymerase chain reaction (PCR) in a nasopharyngeal aspirate sample or a positive serology test. We collected data prospectively between March and July of 2020.
The inclusion criterion was administration hydroxychloroquine, azithromycin, lopinavir/ritonavir or remdesivir for treatment of COVID-19 pneumonia during the period under study, met by a total of 11 cases.
We matched cases for age and sex in a 1:2 ratio to controls without structural heart disease that did not receive any of the drugs under study and without SARS-CoV-2 infection.
For all patients, we collected data on the diagnosis of COVID-19 and pre-existing diseases. When it came to treatment, we recorded the different drugs used and the duration of treatment. Treatment regimens were selected according to the indications established in the protocol of the hospital applicable at the time admission of each patient, which was based on the current recommendations and evidence available at the time.
The standard oral hydroxychloroquine regimen was 6.5 mg/kg/day in children under 6 years or 10 mg/kg/day in children aged 6 or more years to a maximum of 400 mg/day for a total of 5 days. Remdesivir was administered intravenously at a dose of 2.5 mg/kg/day (maximum, 100 mg) for 9 days following a loading dose of 5 mg/kg (maximum, 200 mg) on day 1. Lopinavir/ritonavir was administered orally with a dose of lopinavir of 600 mg/m2/day in infants under 3 months, 24 mg/kg/day in patients with a body weight of up to 15 kg and 20 mg/kg/day in patients with a weight of 15 kg or greater to a maximum of 800 mg/kg/day. Azithromycin was delivered through the oral route at a dose of 10 mg/kg/day for a total of 3 days.
All the electrocardiograms (ECGs) were interpreted manually by the same researcher. The QT interval was measured for 3 different beats, and the corrected QT interval (QTc) was calculated using the Bazett formula (QTc = QT/√ RR). We defined QTc prolongation as a QTc greater than 440 ms in male patients and 460 ms in female patients. The clinical variables analysed were changes in the ECG observed during the administration of the drugs under study relative to baseline. To this end, we established 3 time periods for ECG analysis: baseline before treatment initiation, before 72 h of treatment, and after 72 h of treatment, and we calculated differences in the QTc interval between baseline and the first 72 h of treatment, and between the first 72 h of treatment and after the first 72 h. Due to the challenging circumstances of the time when the study took place, the ECG performed before initiation of treatment was not saved in 2 of the cases.
We have expressed categorical data as proportions and compared them with the F-test. We expressed quantitative data as median and interquartile range (IQR), as the data did not follow a normal distribution. We compared continuous data with the Mann-Whitney U test or the Kruskal-Wallis test. To analyse trends in the QTc interval, we used the Wilcoxon signed-rank test for paired samples. We used a P-value of less than .05 to define statistical significance. The statistical analysis was conducted with the software SPSS version 27.0 (IBM).
ResultsBetween March and December 2020, a total of 48 paediatric patients required admission to our hospital in the context of SARS-CoV-2 infection. Of this total, only 11 received at least one of the drugs under study, corresponding to 22.9% of the total, and all of them managed between the months of March and May. No paediatric patient received these drugs at a later time.
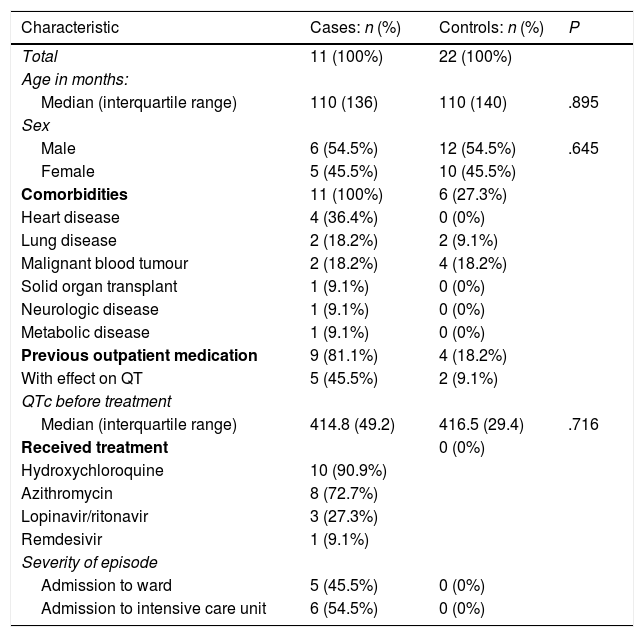
The age of the patients varied widely from 17 days in the youngest and 15 years in the oldest, with a median age of 9 years (IQR, 10.5 years). A total of 45.5% (5/11) were female. Table 1 and Table 2 present the demographic characteristics of the patients.
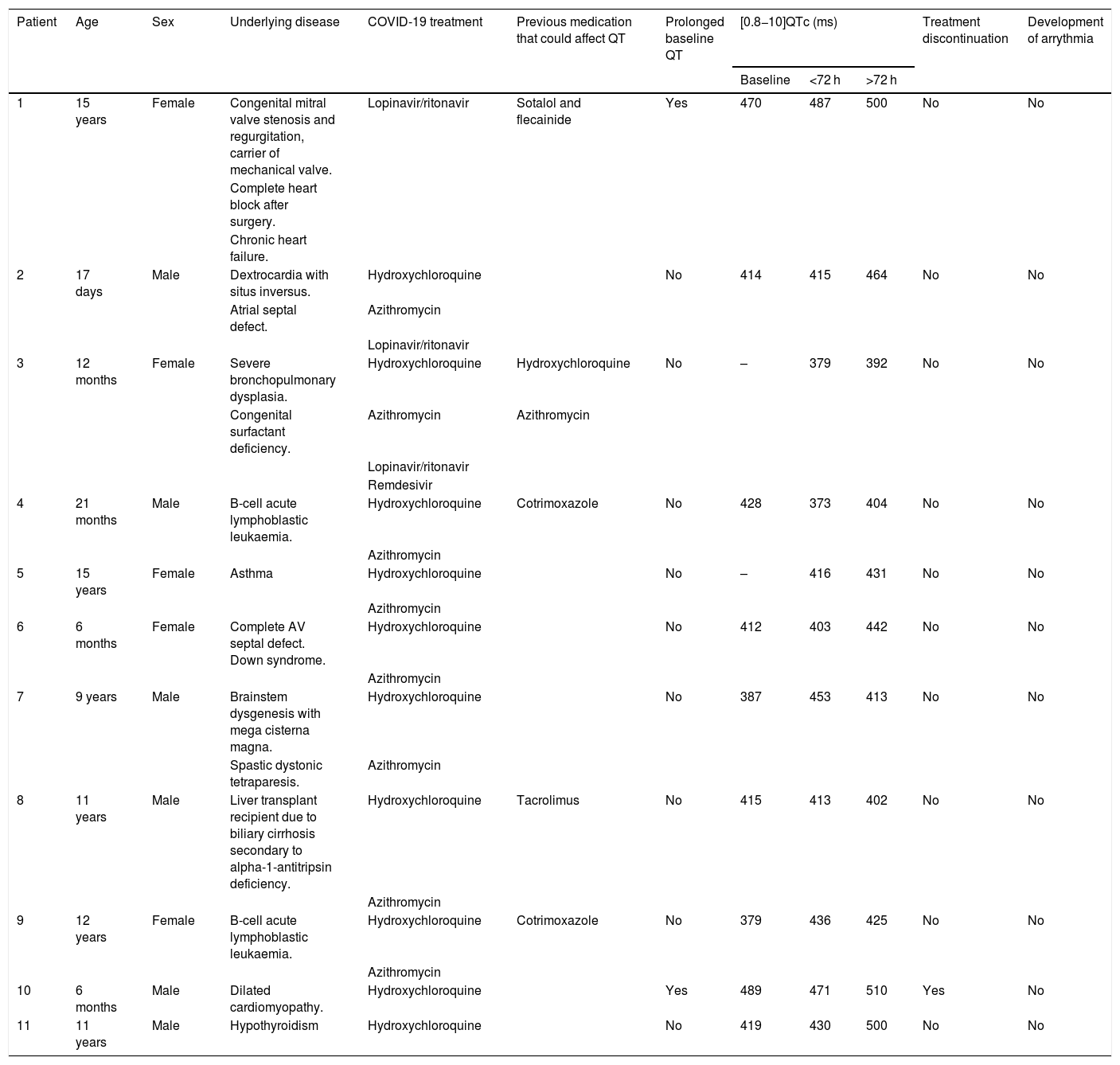
Individual description of the patients included in the study.
| Patient | Age | Sex | Underlying disease | COVID-19 treatment | Previous medication that could affect QT | Prolonged baseline QT | [0.8−10]QTc (ms) | Treatment discontinuation | Development of arrythmia | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | <72 h | >72 h | |||||||||
| 1 | 15 years | Female | Congenital mitral valve stenosis and regurgitation, carrier of mechanical valve. | Lopinavir/ritonavir | Sotalol and flecainide | Yes | 470 | 487 | 500 | No | No |
| Complete heart block after surgery. | |||||||||||
| Chronic heart failure. | |||||||||||
| 2 | 17 days | Male | Dextrocardia with situs inversus. | Hydroxychloroquine | No | 414 | 415 | 464 | No | No | |
| Atrial septal defect. | Azithromycin | ||||||||||
| Lopinavir/ritonavir | |||||||||||
| 3 | 12 months | Female | Severe bronchopulmonary dysplasia. | Hydroxychloroquine | Hydroxychloroquine | No | – | 379 | 392 | No | No |
| Congenital surfactant deficiency. | Azithromycin | Azithromycin | |||||||||
| Lopinavir/ritonavir | |||||||||||
| Remdesivir | |||||||||||
| 4 | 21 months | Male | B-cell acute lymphoblastic leukaemia. | Hydroxychloroquine | Cotrimoxazole | No | 428 | 373 | 404 | No | No |
| Azithromycin | |||||||||||
| 5 | 15 years | Female | Asthma | Hydroxychloroquine | No | – | 416 | 431 | No | No | |
| Azithromycin | |||||||||||
| 6 | 6 months | Female | Complete AV septal defect. Down syndrome. | Hydroxychloroquine | No | 412 | 403 | 442 | No | No | |
| Azithromycin | |||||||||||
| 7 | 9 years | Male | Brainstem dysgenesis with mega cisterna magna. | Hydroxychloroquine | No | 387 | 453 | 413 | No | No | |
| Spastic dystonic tetraparesis. | Azithromycin | ||||||||||
| 8 | 11 years | Male | Liver transplant recipient due to biliary cirrhosis secondary to alpha-1-antitripsin deficiency. | Hydroxychloroquine | Tacrolimus | No | 415 | 413 | 402 | No | No |
| Azithromycin | |||||||||||
| 9 | 12 years | Female | B-cell acute lymphoblastic leukaemia. | Hydroxychloroquine | Cotrimoxazole | No | 379 | 436 | 425 | No | No |
| Azithromycin | |||||||||||
| 10 | 6 months | Male | Dilated cardiomyopathy. | Hydroxychloroquine | Yes | 489 | 471 | 510 | Yes | No | |
| 11 | 11 years | Male | Hypothyroidism | Hydroxychloroquine | No | 419 | 430 | 500 | No | No | |
AV, atrioventricular; QTc, corrected QT interval.
Demographic characteristics of the sample. Comparison of cases and controls.
| Characteristic | Cases: n (%) | Controls: n (%) | P |
|---|---|---|---|
| Total | 11 (100%) | 22 (100%) | |
| Age in months: | |||
| Median (interquartile range) | 110 (136) | 110 (140) | .895 |
| Sex | |||
| Male | 6 (54.5%) | 12 (54.5%) | .645 |
| Female | 5 (45.5%) | 10 (45.5%) | |
| Comorbidities | 11 (100%) | 6 (27.3%) | |
| Heart disease | 4 (36.4%) | 0 (0%) | |
| Lung disease | 2 (18.2%) | 2 (9.1%) | |
| Malignant blood tumour | 2 (18.2%) | 4 (18.2%) | |
| Solid organ transplant | 1 (9.1%) | 0 (0%) | |
| Neurologic disease | 1 (9.1%) | 0 (0%) | |
| Metabolic disease | 1 (9.1%) | 0 (0%) | |
| Previous outpatient medication | 9 (81.1%) | 4 (18.2%) | |
| With effect on QT | 5 (45.5%) | 2 (9.1%) | |
| QTc before treatment | |||
| Median (interquartile range) | 414.8 (49.2) | 416.5 (29.4) | .716 |
| Received treatment | 0 (0%) | ||
| Hydroxychloroquine | 10 (90.9%) | ||
| Azithromycin | 8 (72.7%) | ||
| Lopinavir/ritonavir | 3 (27.3%) | ||
| Remdesivir | 1 (9.1%) | ||
| Severity of episode | |||
| Admission to ward | 5 (45.5%) | 0 (0%) | |
| Admission to intensive care unit | 6 (54.5%) | 0 (0%) |
QTc, corrected QT interval.
One of the salient characteristics of the sample was the presence of significant comorbidities in all 11 patients, diseases that in most cases contributed to the need of admission in the context of infection by SARS-CoV-2. The most frequent underlying diseases were heart diseases (4/11; 36.4%) and diseases resulting immunosuppression, including 2 patients with blood tumours and 1 liver transplant recipient (3/11; 27.3%). Six of the 11 patients (54.5%) required admission to the intensive care unit due to the severity of the disease.
As concerns treatment, 90.9% (10/11) of the patients received hydroxychloroquine, which was the most frequently used drug. In most cases, hydroxychloroquine was combined with azithromycin (8/11 [72.7%]: all but 2 patients treated with hydroxychloroquine), which was the most frequently prescribed combination of drugs. Three patients (27.3%) received lopinavir/ritonavir and 1 (9.1%) remdesivir. The only paediatric patient that received remdesivir was a girl aged 12 months with congenital surfactant deficit that was already receiving hydroxychloroquine and azithromycin for treatment of underlying disease, to which lopinavir/ritonavir and remdesivir were added. None of the patients in the sample received tocilizumab.
Two patients in the COVID-19 case group (2/11; 18.2%) and 2 in the control group (2/22; 9.1%) had QTc prolongation at baseline (>440 ms in male patients and >460 ms in female patients). The median baseline QTc in the case group was 414.8 ms (IQR, 49.2), and we did not find significant differences compared to the control group (median QTc, 416.5 ms; IQR, 29.4) (P = .716). Both cases and controls had a variety of underlying diseases. In fact, 45.5% (5/11) of the group with COVID-19 was in treatment with drugs that affect the QTc interval at baseline: 1 patient was in treatment with sotalol and flecainide, 1 with tacrolimus, 2 with cotrimoxazole and 1, as we noted above, with azithromycin and hydroxychloroquine. All of them were receiving these drugs for long-term medication. In the control group, 2 patients (9.1%) had chronic respiratory diseases and 4 (18.2%) had malignant blood tumours, in 2 cases treated with medication that could affect the QT interval (cotrimoxazole).
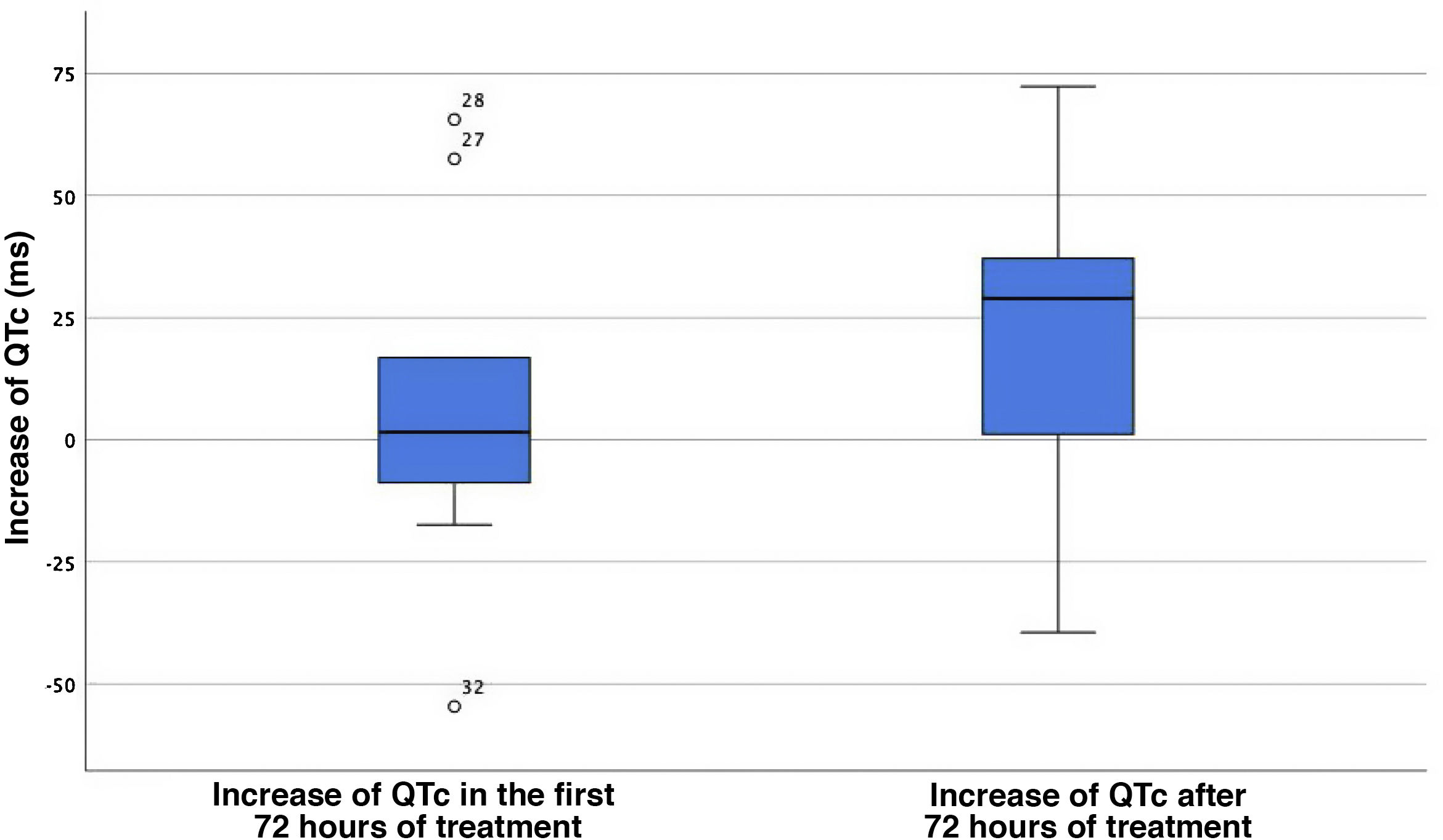
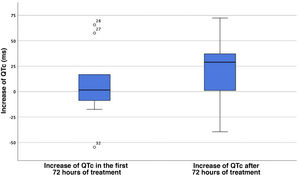
The analysis of QTc changes in the case group revealed a median increase of the QTc of only 1.5 ms (IQR, 50.25) between the baseline ECG and the ECG tracings in the first 72 h of treatment. The median QTc increase starting from 72 h of treatment initiation was 28.9 ms (IQR, 48.7; P = .062) (Fig. 1). Four of the 11 patients with COVID-19 (36.4%) had a prolonged QTc for age, with prolongation present at baseline in 2. Three of them (27.2%) reached a QTc interval of 500 ms or greater, and treatment with hydroxychloroquine was discontinued early in 1 due to a QTc of 510 ms (the other 2 had a QTc of 500 ms). None of the patients experienced severe ventricular arrythmias during the stay and none died.
Increase of the QTc interval in the first 72 h of treatment and after 72 h from treatment initiation. The central value corresponds to the median, and the edges of the box mark the interquartile range. The isolated points with accompanying values are outlier QTc values.
QTc, corrected QT interval.
When it came to the differences between the administered antiviral agents, of the 3 patients that received lopinavir/ritonavir, 2 experienced prolongation of the QTc interval, which was already present at baseline in 1 (this patient was also the only one in the cohort that did not receive hydroxychloroquine). As for remdesivir, the only patient treated with this drug did not exhibit QTc prolongation; this patient also received lopinavir/ritonavir and was under treatment with azithromycin and hydroxychloroquine for underlying disease.
DiscussionAt present, there are very few data on electrocardiographic abnormalities in patients with COVID-19 in the paediatric population. We present an analysis of a series of patients that received antiviral treatment in a tertiary care hospital in Barcelona, Spain (11 of a total of 48 paediatric patients admitted in 2020).
We ought to highlight the substantial proportion of patients with significant comorbidities, a factor that probably plays a role in hospital admission and therefore in pharmacological treatment. In addition to the presence of chronic comorbidities, we ought to mention the high proportion of patients that were receiving medication for underlying conditions, including drugs with an effect on the QTc interval (5/11; 45.5%), and that 2 (18.2%) had a prolonged QTc at baseline. Due to the presence of a number of patients with different diseases in the control group (6/22 [27.3%]; 2/22 [9.1%] treated with drugs that could affect the QTc interval) and the small sample size, we may not be able to rule out the similarity of the baseline QTc in both groups.
When it comes to antiviral agents with arrhythmogenic potential, such as hydroxychloroquine, their long half-lives (approximately 1 month) result in a greater accumulation and higher concentrations of the drug with chronic use compared to short-term use, so shorter courses are probably safer for treatment of COVID-19.10,13
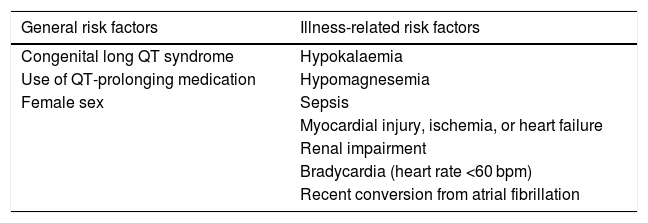
On the other hand, patients with COVID-19 could constitute a group at increased risk of arrhythmia due to the greater incidence of myocardial injury and heart failure associated with the disease.18 In addition, these patients have been found to be more likely to have other risk factors for arrhythmia, such as sepsis, hypoxia, electrolyte imbalances, multiple organ failure and concomitant use of other drugs that prolong the QT interval,10,19 which was also the case in our sample, with a high proportion of patients with chronic diseases and ongoing pharmacological treatment of underlying disease (45.5% were receiving drugs that could affect the QTc interval). Table 3 presents the known risk factors for QT prolongation.10 Severe hypoxia, which has been described in some patients with COVID-19 pneumonia, would also increase the risk of arrhythmia,20 so that it is the patient with most severe disease that are most at risk of developing severe arrhythmias.10 In our sample of 11 patients, none developed arrythmia, despite 54.5% of them having severe enough disease to require admission to the intensive care unit and 2 of them requiring invasive mechanical ventilation.
Risk factors for QT interval prolongation.
| General risk factors | Illness-related risk factors |
|---|---|
| Congenital long QT syndrome | Hypokalaemia |
| Use of QT-prolonging medication | Hypomagnesemia |
| Female sex | Sepsis |
| Myocardial injury, ischemia, or heart failure | |
| Renal impairment | |
| Bradycardia (heart rate <60 bpm) | |
| Recent conversion from atrial fibrillation |
Source: Jankelson et al.10
Due to promising preclinical data, hydroxychloroquine has been the most widely used drug among the different pharmacological treatments tried for management of COVID-19.7,11,13 It has a structure similar to that of quinidine, a class 1A antiarrhythmic agent whose activity prolongs the QT interval and increases the risk of torsades de pointes14. Hydroxychloroquine acts by inhibiting KCNH2 potassium channels (also known as hERG channels), blocking the rapid component of the delayed rectifier potassium current (IKr) and increasing the risk of QTc interval prolongation.19,21
A prolonged QTc interval is the electrocardiographic representation of delayed ventricular repolarization and facilitates the development of early depolarizations that could give rise to torsade de pointes.10 Despite the significant percentage of patients with a prolonged QTc at baseline (2/11; 18.2%) and with treatment (4/11; 36.4%), none developed torsade de pointes or any other form of arrhythmia.
In our study, we found that the largest change in the QTc interval was observed starting from 72 h of treatment, with a median increase of 28.9 ms, although the observed differences were not statistically significant (P = .062). The lack of statistical significance is probably due to the small size of the sample, with was the main limitation of the study. Multicentre studies in larger samples would be useful to confirm this association, although the removal of these drugs from the COVID-19 treatment protocols poses a barrier to their performance. To date, no studies have been published on the use of hydroxychloroquine in the context of the pandemic in sizeable samples of paediatric patients, and the only available data come from small cohort studies,16 so our case series is one of the few sources in the literature on its use in paediatric patients in Spain.
Many of the protocols for treatment of the novel coronavirus have included the use of azithromycin combined with hydroxychloroquine. Azithromycin also affects the QT interval through inhibition of the IKr current,22,23 and it is hypothesised that there is an increased risk of QTc in patients treated with the combination of these 2 drugs, in addition to an increased risk of bundle branch block.9 In addition, the IKr current could also be affected in patients with SARS-CoV-2 infection due to the increased production of interleukin-6.24 Given the scarcity of the evidence supporting the beneficial effects of hydroxychloroquine and its combination with azithromycin, some authors recommend trying to avoid their use.25
Other drugs used for treatment of SARS-CoV-2, such as favipiravir or lopinavir/ritonavir, can also result in QT interval prolongation and torsade de pointes.13 The combination of several drugs that may affect the QT interval can have a particularly significant effect on patients under chronic treatment with drugs that affect the QT interval, as was the case of the patient treated with sotalol and flecainide with a prolonged baseline QTc.
Since patients who require admission usually have multiple comorbidities and may be receiving several drugs for treatment of underlying diseases, and given the potential effect on the QTc that we observed in the drugs under study, clinicians should carefully consider the risks and benefits of their use and closely monitor the QTc interval. Several publications offer guidance on the monitoring of QT interval prolongation in patients with COVID-19 treated with hydroxychloroquine. Their recommendations are not entirely consistent, with some authors proposing ECG monitoring at baseline and during treatment10,13,26 and others reserving this recommendation for at-risk patients,27 while the main barriers to ECG monitoring identified in the literature are the heavy workloads of clinicians and the increased exposure to infected patients associated with this approach.26
When it comes to paediatric patients, it seems reasonable to always do an ECG before initiating treatment and to monitor patients with repeated ECGs, mainly starting from 72 h of treatment, to assess whether they have developed substantial QTc prolongation and suspend treatment in patients with sustained QTc values greater than 500 ms.
Conflicts of interestThe authors have no conflicts of interest to declare.
Data availabilityData will be made available on request.
Please cite this article as: Esmel-Vilomara R, Dolader P, Sabaté-Rotes A, Soriano-Arandes A, Gran F, Rosés-Noguer F. Evolución del intervalo QTc en pacientes con infección SARS-COV-2 tratados con farmacos antivirales. An Pediatr (Barc). 2022;96:213–220.
Previous presentations: The results of the study were submitted to the 54th Annual Meeting for European Paediatric and Congenital Cardiology held in May 25–27, 2021 in Goteborg.