Short stature is the most frequent reason for consultation in Pediatric Endocrinology consultations and sometimes requires treatment with growth hormone. The aim of the study was to analyze the response to treatment based on its onset in pubertal or prepubertal stages and to analyze the possible benefit of an early onset.
Patients and methodsLongitudinal, retrospective and observational study in 139 patients treated for idiopathic growth hormone deficiency up to adult height. Main variables studied: (a) genetic background: maternal, paternal and genetic height; (b) perinatal history; (c) anthropometry during follow-up and at pubertal onset: weight, height, body mass index; (d) variables during follow-up and at pubertal onset: growth rate, bone age and growth prognosis. Final response variables: adult height, adult height with respect to target height, adult height with respect to initial growth prediction, adult height with respect to initial height at the start of treatment and adult height with respect to height at pubertal onset.
ResultsTotal pubertal gain was 0.84 ± 0.6 SD. 61.9% of the patients started treatment with rhGH in prepuberty. The initiation of treatment in the prepubertal stage and a higher total pubertal gain are correlated with a better final height (P = 0.001 and r = 0.507, P = 0.00, respectively). Furthermore, a longer duration of treatment in pre-puberty is correlated with a better final response (r = 0.328, P = 0.00).
ConclusionsThe start of treatment in the prepubertal stage and its longer duration during this period are determining factors to achieve a good long-term response. Total pubertal gain was greater in patients who started treatment in the pubertal stage.
La talla baja es motivo de consulta frecuente en Endocrinología Pediátrica, precisando en ocasiones tratamiento con hormona del crecimiento (GH). El objetivo del estudio fue analizar la respuesta al tratamiento en función de su inicio en la etapa puberal o prepuberal y analizar el posible beneficio de un comienzo precoz.
Pacientes y métodosEstudio longitudinal, retrospectivo y observacional en 139 pacientes tratados por déficit de GH idiopático (grave o parcial) hasta talla adulta. Principales variables estudiadas: a) antecedentes familiares: talla materna, paterna y genética; b) antecedentes perinatales; c) antropometría durante el seguimiento y al inicio puberal: peso, talla, índice de masa corporal, y d) variables durante el seguimiento y al inicio puberal: velocidad de crecimiento, edad ósea y pronóstico de crecimiento. Variables de respuesta final: talla adulta, talla adulta respecto a talla genética, talla adulta respecto al pronóstico de crecimiento inicial, talla adulta respecto a talla al inicio del tratamiento y talla adulta respecto a talla al inicio puberal.
ResultadosLa ganancia puberal total fue de 0,84 ± 0,6 DE. Un 61,9% de los pacientes iniciaron tratamiento con GH en prepubertad. El inicio del tratamiento en la etapa prepuberal y una mayor ganancia puberal total se relacionaron con una mejor talla final (p = 0,001, y r = 0,507, p = 0,00 respectivamente). Además, una mayor duración del tratamiento en la prepubertad se correlacionó con una mejor respuesta final (r = 0,328, p = 0,00).
ConclusionesEl inicio del tratamiento en la prepubertad y una mayor duración durante este periodo son factores determinantes para alcanzar una mejor respuesta a largo plazo. La ganancia puberal total fue mayor en los pacientes que iniciaron el tratamiento en etapa puberal.
Puberty and growth are very important physiological phenomena that entail vast changes.1 An increase in the secretion of gonadal steroid hormones triggers the onset of the pubertal growth spurt.2 It determines the growth velocity (GV) in puberty, sexual maturation and the age at menarche.3 Normal puberty starts from age 9 years with a testicular volume of 4 mL or greater measured with a Prader orchidometer in boys, and the development of breast buds in girls.4 Pubertal growth is important because it accounts for approximately 25% of total postnatal growth, slightly more in men.5 Abnormalities in pubertal growth are a frequent and clinically relevant reason for seeking medical care.1
Short stature is the most frequent presenting complaint in paediatric endocrinology practice.6 Short stature is defined as a height more than 2 standard deviations below the mean for age and sex in the reference population.7
Growth hormone deficiency (GHD) is an important cause of short stature in the paediatric population.8 It manifests differently depending on the stage of development when onset occurs (early after birth, during childhood, during adolescence or in adulthood), the underlying cause or mechanism (genetic, acquired or idiopathic), the severity and whether growth hormone (GH) is the sole affected pituitary hormone, other pituitary hormones are involved or the deficiency is part of a complex syndrome.9 There is ample evidence that early diagnosis and treatment of GHD are key due to its impact on neurodevelopmental, metabolic and anthropometric outcomes.8
The main goal of treatment with recombinant human growth hormone rhGH in these patients is to normalise linear growth during childhood and adolescence and achieve an adult height (AH) in the normal range and consistent with the genetic potential or target height (TH).10,11 Different clinical trials have demonstrated the efficacy of rhGH treatment for catch-up growth.7
Multiple studies suggest that initiation of treatment with rhGH at an early age increases the likelihood that patients will reach an AH consistent with the TH. Thus, treatment should start as early as possible to maximize the growth response.10,12 Other authors have proposed that the cumulative exposure to rhGH in prepubertal years is a significant factor and have found a correlation between linear growth in the prepubertal stage and total linear growth, which evinces the importance of initiating treatment before the onset of puberty.13,14
In this context, the objective of our study was to assess the response to treatment based on whether it was initiated before or after the onset of puberty, assess the potential correlation between initiation of treatment in the prepubertal stage and improved final height outcomes and, in patients that started treatment in the prepubertal stage, the possible benefits of early initiation of treatment.
Patients and methodsStudy populationWe conducted a longitudinal, retrospective and observational study in patients followed up in the paediatric endocrinology clinic of a tertiary care hospital born between 1989 and 2004 that underwent treatment for idiopathic GHD. We defined severe idiopathic GHD as a peak GH concentration in the GH stimulation test of less than 3 ng/mL and partial GHD as a peak GH concentration in the test of 3−10 ng/mL.
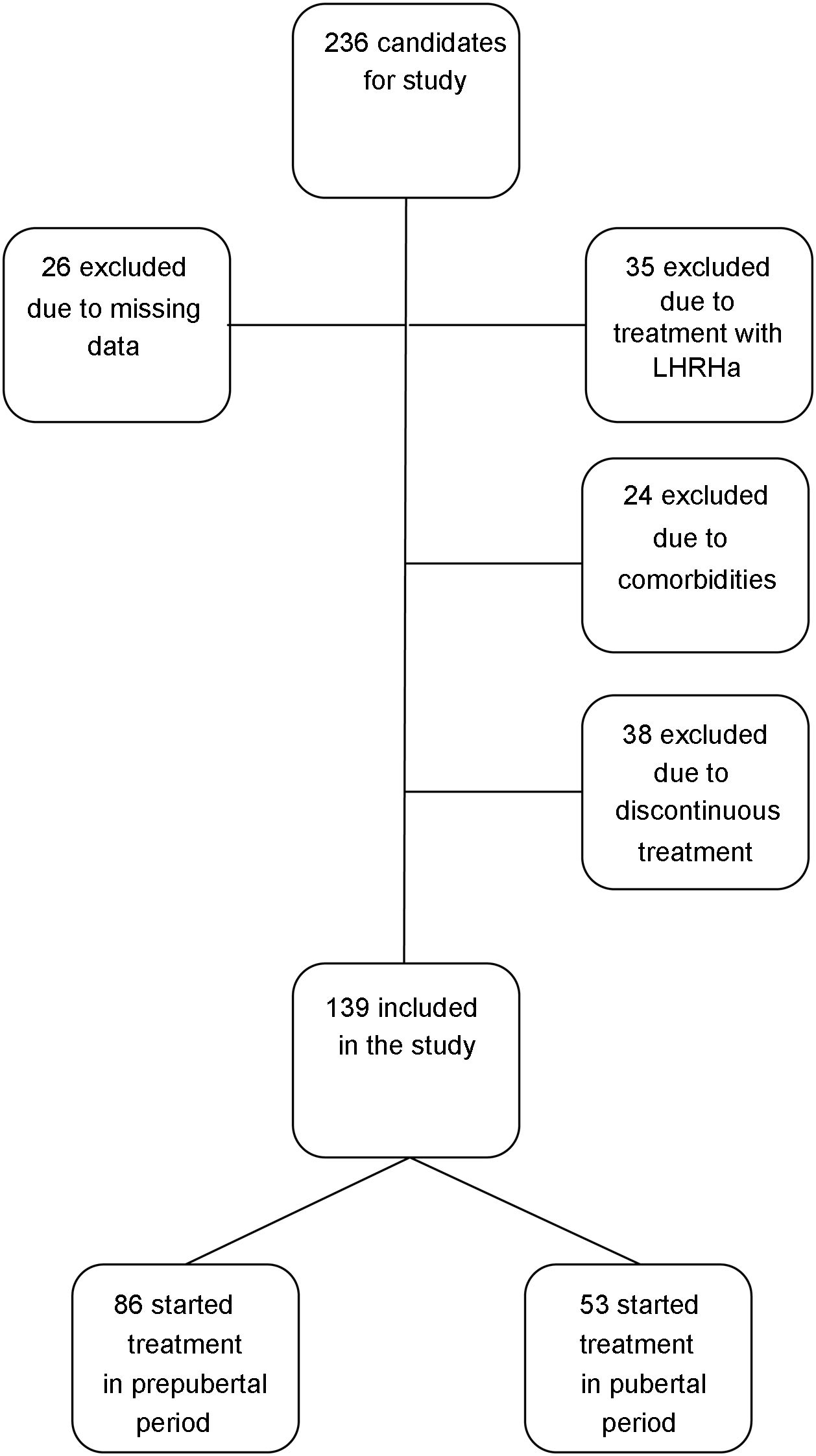
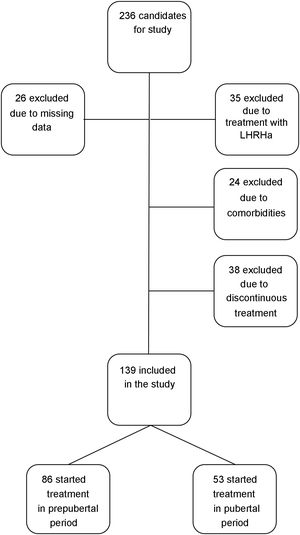
We included patients with a height more than 2 SDs below the mean for age and sex at diagnosis of GHD, and patients with peak GH concentrations under 10 ng/mL in 2 GH stimulation tests treated with rhGH at replacement doses for at least 1 year who reached their near-final adult heights during the follow-up. We defined near-final adult height as a growth velocity of less than 0.5 cm/years and a bone age of 15 years in female patients or 17 years in male patients. We used this concept in reference to AH because patients were discharged before reaching their final height. We excluded patients with GHD of organic aetiology, patients with comorbidities that could affect growth and height outcomes (cancer, congenital heart disease, cerebral palsy, chromosome disorders or genetic syndromes), patients that received concomitant treatment with luteinising hormone-releasing hormone analogues, patients that received a diagnosis of GHD with a single stimulation test or patients that received discontinuous treatment with rhGH, amounting to a total of 97 patients (n = 97), so that the final sample included 139 patients (n = 139). We did not exclude any patients due to poor response to treatment or development of adverse events (Fig. 1).
Data collectionWe analysed the following variables: 1) genetic potential: maternal height, paternal height and TH; 2) perinatal history: type of pregnancy, gestational age at birth, type of delivery, perinatal disease and neonatal anthropometric measurements; 3) anthropometric measurements during follow-up: weight, height, body mass index (BMI); 4) anthropometric measurements at onset of puberty (defined as thelarche in female patients and a testicular volume ≥4 mL in male patients): weight, height, BMI; 5) follow-up variables: GV, bone age, predicted height (PH) and AH; 6) variables at onset of puberty: GV, bone age and PH; 7) laboratory test results: analysis of components of GH axis-insulin-like growth factor (IGF) (peak GH concentration after GH stimulation test), IGF type 1 (IGF-1), Insulin-like growth factor binding protein 3 (IGFBP3) and 8) treatment: rhGH dose (µg/kg/day).
We expressed the height, weight and BMI variables as absolute values and as z-scores using the growth tables of the 2010 Growth Studies of the Spanish population as reference.15 We expressed GV in absolute terms and as a z-score using the 2010 Spanish Growth Studies tables as reference.16 We calculated bone age using the Radiographic atlas of skeletal development atlas published by Greulich and Pyle17 and calculated the predicted adult height (PH) based on current height and bone age applying the method proposed by Bayley and Pinneau.18 We assessed pubertal development applying the Tanner criteria. To make a comparative assessment of growth during puberty, we used the published longitudinal data for normal Spanish children from birth through adulthood.19 We recorded and analysed all these variables at 1 year before treatment initiation, at initiation of treatment with rhGH, at onset of puberty and yearly until the patient reached the near-final AH.
We used the following endpoints to assess the overall response to treatment:
- a
AH (z-score).
- b
Difference between AH and TH (z-score).
- c
Difference between AH and initial PH (z-score).
- d
Difference between AH and height at treatment initiation (z-score).
- e
Difference between AH and height at onset of puberty (z-score).
We assessed the effectiveness of rhGH for treatment of GHD in terms of the AH and the response to treatment based on whether it was initiated before or after onset of puberty to analyse the potential correlation of initiation during the prepubertal stage with improved final outcomes. We also analysed the subset of patients that started treatment in the prepuberal stage and assessed whether earlier initiation of treatment during the prepubertal stage was associated with improved final outcomes.
Statistical analysisWe assessed the normality of the data before the statistical analysis, and then proceeded to compare means and assess correlations. To analyse the association between categorical variables, we used the chi square test. TO assess the association between quantitative variables, we used the Pearson correlation coefficient. TO assess the association between quantitative and categorical variables, we used the Student t test. The statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) version 15.0, defining statistical significance as a p-value of less than 0.05.
The study was approved by the Research Ethics Committee of the Autonomous Community of Aragon, Spain.
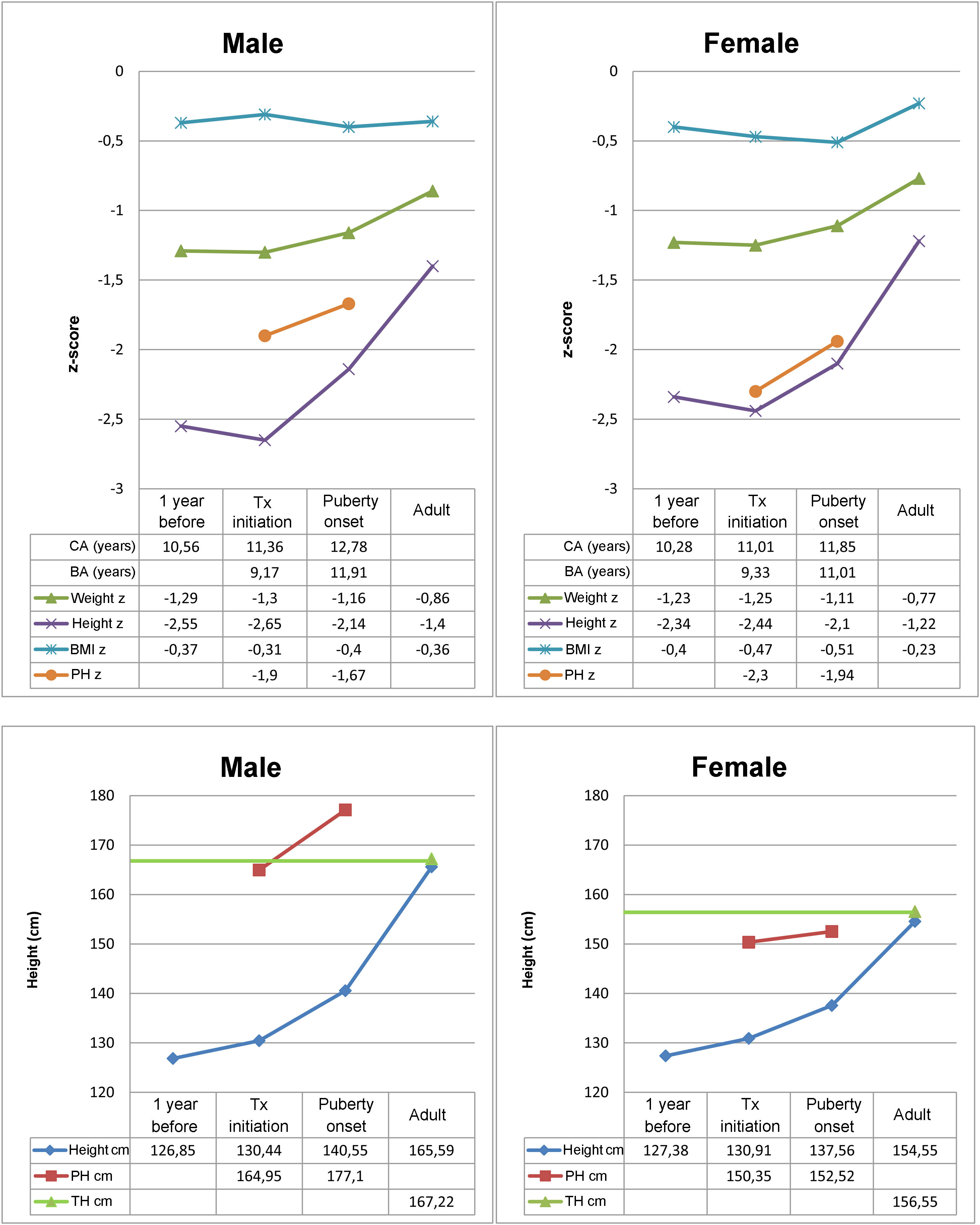
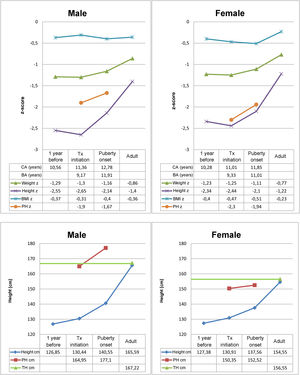
ResultsThe sample included 139 patients (96 female and 43 male). Of this total, 71.2% had severe GHD and 28.2% partial GHD. The mean concentration of GH in stimulation tests at diagnosis was 4.04 ± 2.57 ng/mL (0 ng/mL–9.86 ng/mL). The mean age at treatment initiation was 11.1 ± 2.5 years, with a mean duration of treatment of 4.2 ± 2 years, and a mean initial dose of 26.14 ± 2 µg/kg/day in the total sample, 26.02 ± 2.52 µg/kg/day in patients that started treatment before puberty and of 26.33 ± 2.14 µg/kg/day in patients that started it after the onset of puberty, without significant differences between the two groups (P = 0.454). The doses were similar during pubertal development. The mean age at the end of treatment was15.3 ± 1.2 years. The mean TH z-score was –1.3 ± 0.7, the mean initial height z-score –2.5 ± 0.4 and the mean initial PH z-score –2.19 ± 0.6, that is, 150.35 ± 3.40 cm for female patients and 164.9 ± 2.87 cm for male patients. The mean age at menarche was 13.67 ± 1 years.
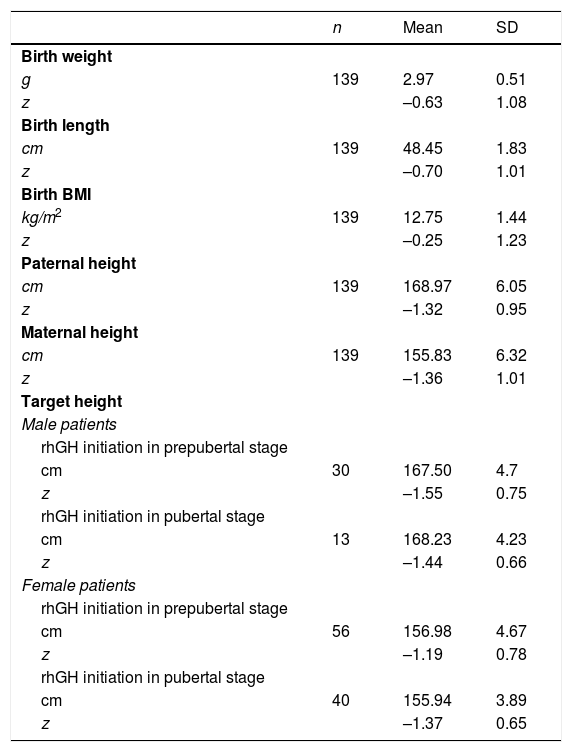
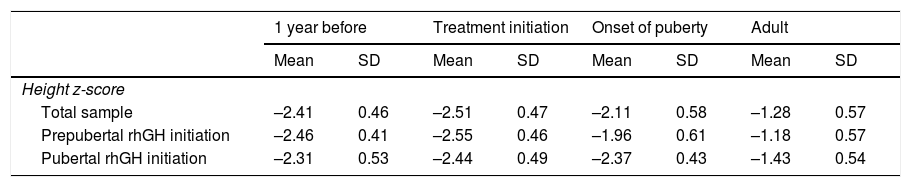
Table 1 summarises birth data, parental heights and THs in the sample. Fig. 2 presents the results of the descriptive analysis of patient characteristics and outcomes during treatment. Table 2 compares height outcomes in patients that started treatment before and after the onset of puberty.
Birth characteristics, parental heights and target heights expressed as absolute values and z-scores.
| n | Mean | SD | |
|---|---|---|---|
| Birth weight | |||
| g | 139 | 2.97 | 0.51 |
| z | –0.63 | 1.08 | |
| Birth length | |||
| cm | 139 | 48.45 | 1.83 |
| z | –0.70 | 1.01 | |
| Birth BMI | |||
| kg/m2 | 139 | 12.75 | 1.44 |
| z | –0.25 | 1.23 | |
| Paternal height | |||
| cm | 139 | 168.97 | 6.05 |
| z | –1.32 | 0.95 | |
| Maternal height | |||
| cm | 139 | 155.83 | 6.32 |
| z | –1.36 | 1.01 | |
| Target height | |||
| Male patients | |||
| rhGH initiation in prepubertal stage | |||
| cm | 30 | 167.50 | 4.7 |
| z | –1.55 | 0.75 | |
| rhGH initiation in pubertal stage | |||
| cm | 13 | 168.23 | 4.23 |
| z | –1.44 | 0.66 | |
| Female patients | |||
| rhGH initiation in prepubertal stage | |||
| cm | 56 | 156.98 | 4.67 |
| z | –1.19 | 0.78 | |
| rhGH initiation in pubertal stage | |||
| cm | 40 | 155.94 | 3.89 |
| z | –1.37 | 0.65 | |
BMI, body mass index; SD, standard deviation.
Differences in mean values and standard deviations of the height z-score based whether treatment with rhGH started before or after onset of puberty.
| 1 year before | Treatment initiation | Onset of puberty | Adult | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Height z-score | ||||||||
| Total sample | –2.41 | 0.46 | –2.51 | 0.47 | –2.11 | 0.58 | –1.28 | 0.57 |
| Prepubertal rhGH initiation | –2.46 | 0.41 | –2.55 | 0.46 | –1.96 | 0.61 | –1.18 | 0.57 |
| Pubertal rhGH initiation | –2.31 | 0.53 | –2.44 | 0.49 | –2.37 | 0.43 | –1.43 | 0.54 |
SD, standard deviation.
A total of 61.9% patients started treatment with rhGH before the onset of puberty (40.3% of female patients and 21.6% of male patients), with a mean chronological age at treatment initiation of 9.87 ± 1.9 years in female patients and 10.14 ± 3.2 years in male patients. The mean time elapsed from initiation of rhGH to onset of puberty was 1 ± 2.2 years (1.4 ± 2.9 years in male patients and 0.8 ± 1.7 years in female patients). In the 38.1% of patients that started treatment after the onset of puberty, the mean age at initiation was 12.6 ± 0.9 years in female patients and 14.19 ± 1.4 years in male patients.
Patients reached AHs corresponding to z-scores that exceeded the baseline height z-scores relative to the reference population (AHz of –1.28 ± 0.6 vs initial height z of –2.5nbsp;± 0.4), with a mean AH of 154.5 ± 3.7 cm in female patients and 165.6 ± 4.1 cm in male patients. The difference between the AH and the TH z-scores was of 0.06 ± 0.7 and the difference between the AH and initial PH z-scores 0.9 ± 0.6. We found an increase of the AH relative to the initial height of 1.2 ± 0.6 standard deviations, corresponding to increases of +0.4 ± 0.5 in the prepubertal period and +0.84 ± 0.6 after the onset of puberty, without significant differences based on sex. The AH z-score was 0.57 ± 0.6 points greater compared to the PH at the onset of puberty. The total pubertal height gain (TPHG) was of 16.96 ± 3.9 cm in female patients and 25.04 ± 5.3 cm in male patients. Pubertal height gain was greater in patients that started treatment after the onset of puberty.
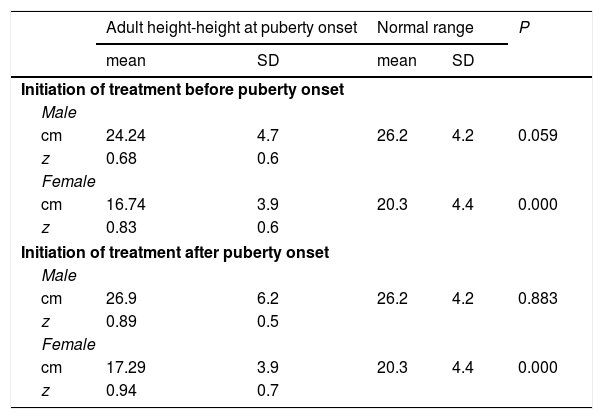
Linear growth after the onset of puberty was comparable to that in the reference population in male patients (25.04 ± 5.3 cm vs 26.2 ± 4.2 cm), with a tendency towards lesser growth (albeit not statistically significant) in patients that started treatment before puberty (24.24 ± 4.7 cm vs 26.2 ± 4.2 cm; P = 0.059). However, the height gain relative to the reference population was smaller in female patients (16.96 ± 3.9 cm vs 20.3 ± 4.4 cm), with statistically significant differences in the subset that started treatment before the onset of puberty relative to the reference population (16.74 ± 3.9 cm vs 20.3 ± 4.4 cm; P = 0.00) and to the group that started treatment after the onset of puberty (17.29 ± 3.9 cm vs 20.3 ± 4.4 cm; P = 0.00) (Table 3). We found a direct and significant correlation between TPHG and AH (r = 0.507; P = 0.00).
Mean values and standard deviation of total pubertal linear growth based on the timing of rhGH initiation.
| Adult height-height at puberty onset | Normal range | P | |||
|---|---|---|---|---|---|
| mean | SD | mean | SD | ||
| Initiation of treatment before puberty onset | |||||
| Male | |||||
| cm | 24.24 | 4.7 | 26.2 | 4.2 | 0.059 |
| z | 0.68 | 0.6 | |||
| Female | |||||
| cm | 16.74 | 3.9 | 20.3 | 4.4 | 0.000 |
| z | 0.83 | 0.6 | |||
| Initiation of treatment after puberty onset | |||||
| Male | |||||
| cm | 26.9 | 6.2 | 26.2 | 4.2 | 0.883 |
| z | 0.89 | 0.5 | |||
| Female | |||||
| cm | 17.29 | 3.9 | 20.3 | 4.4 | 0.000 |
| z | 0.94 | 0.7 | |||
Expressed as cm and z-score for each sex, reference data obtained from the study Estudio longitudinal de niños españoles normales desde el nacimiento hasta la edad adulta.
Statistical significance defined as P < 0.05.
SD, standard deviation.
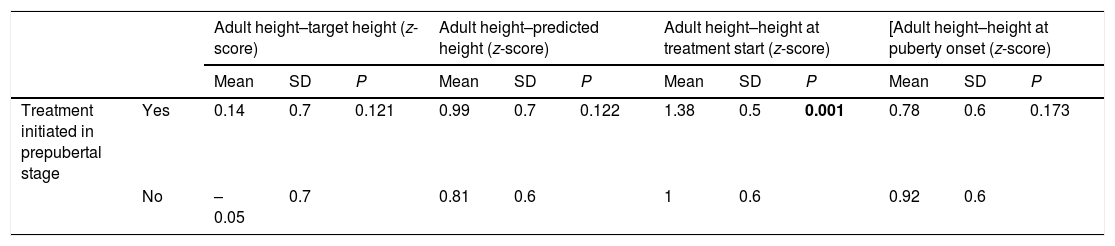
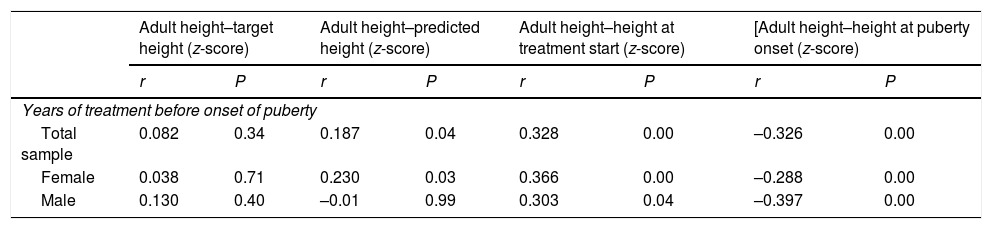
Both patients that started treatment in the prepubertal period (n = 86; AHz–THz = 0.14 ± 0.7) and those that started after puberty onset (n = 53; AHz–THz = –0.05 ± 0.7) reached their TH, with no significant differences between the groups (P = 0.126). Initiation of treatment in the prepuberal period was associated with better final outcomes (AH compared to initial height; P = 0.001) (Table 4). Furthermore, longer treatment in the prepubertal stage was significantly associated with better linear growth outcomes (Table 5).
Association between treatment endpoints and initiation of treatment before or after the onset of puberty.
| Adult height–target height (z-score) | Adult height–predicted height (z-score) | Adult height–height at treatment start (z-score) | [Adult height–height at puberty onset (z-score) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | P | Mean | SD | P | Mean | SD | P | Mean | SD | P | ||
| Treatment initiated in prepubertal stage | Yes | 0.14 | 0.7 | 0.121 | 0.99 | 0.7 | 0.122 | 1.38 | 0.5 | 0.001 | 0.78 | 0.6 | 0.173 |
| No | –0.05 | 0.7 | 0.81 | 0.6 | 1 | 0.6 | 0.92 | 0.6 | |||||
Statistical significance defined as P < 0.05.
Statistically significant results are presented in boldface.
SD, standard deviation.
Correlation between the years of treatment before the onset of puberty and treatment endpoints in the group that started treatment in the prepubertal period.
| Adult height–target height (z-score) | Adult height–predicted height (z-score) | Adult height–height at treatment start (z-score) | [Adult height–height at puberty onset (z-score) | |||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Years of treatment before onset of puberty | ||||||||
| Total sample | 0.082 | 0.34 | 0.187 | 0.04 | 0.328 | 0.00 | –0.326 | 0.00 |
| Female | 0.038 | 0.71 | 0.230 | 0.03 | 0.366 | 0.00 | –0.288 | 0.00 |
| Male | 0.130 | 0.40 | –0.01 | 0.99 | 0.303 | 0.04 | –0.397 | 0.00 |
Statistical significance defined as P < 0.05.
r, Pearson correlation coefficient.
Our study included 139 patients with GHD treated with rhGH for a minimum of 1 year that reached the near-final AH, which allowed us to analyse linear growth outcomes in a substantial number of patients. More than half the patients were female, a percentage similar to the proportions described in previous studies, including those published by Ranke et al.,20,21 Rachmiel et al.22 or Straetemans et al.23 but greater compared to other studies in which male patients predominated, such as the studies performed by Carrascosa et al.24,25 or Ariza Jiménez et al.26 This may be due to the size of the sample under study, and the sex distribution would probably be uniform if the sample were larger. However, the fact that we found a greater proportion of female patients highlights the importance of early diagnosis, as girls experience the onset of puberty earlier and reach the final height compared to boys, which has an impact on the potential total duration of treatment.
There is ample evidence of the positive impact of treatment with rhGH on GV,7,10,11 which was corroborated by our findings, as treatment with rhGH in our sample was effective in achieving AHs in the normal range consistent with the TH. Other aspects that are important in research on this subject are the assessment of adherence to treatment and the inclusion of other parameters to make a more comprehensive assessment.27
The age at onset of puberty in the male patients included in the sample was similar to the age reported in previous studies,3,19,28 except the study by Bundak et al.,29 in which boys experienced the onset of puberty earlier. In contrast, female patients in the sample experienced the onset of puberty at a later age compared to the previous literature,3,4,19 in which the age at onset of puberty is approximately 10 years, except for the study by Maghnie et al.28 in which the mean age was 12.6 years. The bone age at onset of puberty was consistent with the bone age reported in the studies by García-Cuartero et al.,4 Ferrández Longás et al.19 and Maghnie et al.28 The height and BMI of female patients in our study at onset of puberty were similar to those in the reviewed literature.3,4,28 Male patients in the sample had smaller heights compared to those reported by García Cuartero et al.,4 Maghnie et al28 and Bundak et al.,29 of around 147 cm, although the BMI values were similar. The mean age at menarche in our study was greater compared to the findings of longitudinal studies conducted by Carrascosa et al. (12.9 ± 1.1)3 and Ferrández Longás et al. (12.6 ± 0.9).19 The delay in the onset of puberty and menarche in female patients, which occurred later compared to longitudinal studies in healthy children, may be related to the pathophysiology of GHD, as the disease is characterised by proportionate decreased postnatal growth, including delayed puberty30 and delayed bone age31. However, although the age at onset of puberty and at menarche in female patients in the sample were greater compared to other case series, we ought to highlight that the time elapsed between the onset of puberty and menarche was consistent with the previous literature and with the reference population.3,19
Many studies suggest that initiation of treatment with rhGH at an early age helps patients achieve an AH in the range of their TH. Thus, treatment should be started as soon as possible to maximise linear growth outcomes.10,12 We found that 61.9% of patients had started treatment with rhGH before puberty at a chronological age at treatment initiation that was similar to those reported in studies like the one conducted by Ranke y Lindberg32 or the Kabi International Growth Study (KIGS)33 in both sexes. However, 38.1% started treatment after the onset of puberty, as the earliest patients included in the sample were treated long ago and started treatment when it was first authorised for management of GHD, and this percentage does not reflect current clinical practice, as diagnosis is made at younger ages, but the analysis of this subset is useful to compare responses to treatment based on the timing of initiation. In fact, we ought to highlight that we found a progressive decrease in the age at initiation of treatment from the earliest cases in the sample to the most recent ones.
In our study, the TPHG was similar to those reported by Maghnie et al.28 and Bundak et al.,29 but greater compared to the study by García-Cuartero et al.4 Based on our data, a higher TPHG is associated with greater AH outcomes, which supports the findings of other authors, including Ariza Jiménez et al.26 and Maghnie et al.28 A finding in our study that has not been described by previous authors was that the TPHG was greater in patients that started treatment in the pubertal period, which demonstrated the impact of puberty on final height. A possible explanation is that patients that started treatment in the prepubertal period had some catch-up growth before the onset of puberty, whereas those that started in the pubertal period had not yet started the catch-up growth and therefore had higher height gains during puberty.
The duration of treatment in the prepubertal period was considerably shorter compared to the duration reported by Carrascosa et al.3 in their longitudinal study, which was of 3.6 years. A possible explanation is that, as we mentioned before, some of the patients in our study were treated a long time ago.
Many studies have found an association between initiation of treatment before puberty onset and improved final height outcomes. The study conducted by Patel et al.13 found that younger age at treatment initiation and initiation in the prepubertal period were associated with better outcomes in patients with GHD. Consistent with the hypothesis that sensitivity to rhGH is greater during childhood and that treatment efforts to improve final height outcomes should focus on the prepubertal period, Root et al.12 found that catch-up growth in these patients took place during childhood, before the onset of puberty. The findings of Reiter et al.14 suggested that total exposure to rhGH in the prepubertal years could be a significant contributor to improved height outcomes, as the height gain in the prepubertal stage was strongly correlated to total height gain, thus confirming the importance of initiating treatment before puberty. Furthermore, in a study conducted more recently, Cavarzere et al.34 concluded that GH secretion should be re-evaluated mid-puberty to reduce potential adverse events and minimise costs without affecting final height outcomes. The effectiveness and cost-effectiveness of treatment with rhGH are therefore greater in the prepuberal period.10,32 Thus, as was also the case in our study, early initiation of treatment in the prepubertal period has been associated with better final height outcomes. However, our study included patients that started treatment in the prepubertal as well as pubertal periods, and found an association between the final height and initiation of treatment in the prepubertal period, assessed by the difference between the AH and the initial height. What our study contributes is that diagnosis of GHD in puberty should not limit treatment with rhGH, as the response to treatment was still adequate, although it is reasonable to assume that due to the shorter duration of treatment, the heigh gain will not be as great as if treatment had been initiated before the onset of puberty.
The study by Ranke et al.,20 to be interpreted taking into account the fact that the growth velocity decreases with age during the prepubertal period, found that in the group of patients that started treatment before the onset of puberty the response to rhGH was better in the youngest patients. Similarly, in the subset of patients with prepubertal initiation of treatment in our study, a longer duration of treatment before the onset of puberty was associated with better AH outcomes.
In conclusion, our findings reveal that patients that start treatment before the onset of puberty had better final height outcomes. In addition, the earlier treatment starts and the longer the duration of treatment in the prepubertal period, the better the outcome of treatment, although diagnosis of GHD after the onset of puberty does not preclude treatment with rhGH, as it can also achieve adequate outcomes. Furthermore, greater pubertal height gains lead to better final height outcomes. Our results corroborate the findings of previous studies and are relevant because they can guide clinical decision-making as regards the optimal timing for treatment initiation in order to maximise its benefits.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sánchez Malo MJ, Hidalgo Sanz J, Hernández Tejedor C, García Ventura M, Ferrer Lozano M, Labarta Aizpún JI, et al. Déficit de hormona de crecimiento: influencia de la pubertad en la respuesta al tratamiento. An Pediatr (Barc). 2022;96:221–229.