Pulmonary atresia with intact ventricular septum and critical pulmonary stenosis in newborns encompasses a wide spectrum of disease, including cases with significant right ventricular hypoplasia and coronary artery to right ventricle fistulae, which may be considered a contraindication for decompression of the right ventricle. The aim of this study was to review the middle- and long-term outcomes of these patients over 20 years and identify differential factors between both groups, including patients with coronary artery fistulae.
Patients and methodsWe performed a descriptive retrospective study by identifying all patients that received a diagnosis of pulmonary atresia with intact ventricular septum and critical pulmonary stenosis between January 1996 and January 2018. We collected and analysed data regarding right ventricular morphology, surgical management, percutaneous intervention and medium- and long-term outcomes.
Results51 patients were admitted. A total of 9 patients (17.6%) died during the followup. None of the deceased patients had coronary artery to right ventricle fistulae. The median length of followup in the 42 survivors was 107.81 months (±53.56).
The functional class based on the latest revision of the New York Heart Association classification was 1.2 for the overall sample. Survivors of critical pulmonary stenosis had a functional class of 1.1, and survivors of pulmonary atresia with intact ventricular septum a functional class of 1.6. There were no differences based on the presence or absence of coronary artery to right ventricle fistulae.
ConclusionsCoronary artery to right ventricle fistulae may not be a contraindication for biventricular strategy. Patients with critical pulmonary stenosis had better outcomes compared to patients with pulmonary atresia with intact ventricular septum. The aggressive strategy of opening the pulmonary valve early on was associated with a good overall survival and correlated to a good functional class.
Los neonatos afectos de atresia pulmonar con tabique interventricular íntegro y estenosis pulmonar crítica representan un espectro amplio, incluyendo aquellos con hipoplasia significativa del ventrículo derecho. La presencia de fístulas arteriales coronarias a ventrículo derecho puede ser una contraindicación para la descompresión del ventrículo derecho. El principal objetivo del presente trabajo es analizar los resultados a corto y largo plazo durante 20 años de estos pacientes, e identificar los factores diferenciales entre ambos grupos incluyendo aquellos pacientes afectos por fístulas arteriales coronarias.
Pacientes y métodosEste un estudio retrospectivo donde se identificaron todos los pacientes diagnosticados de atresia pulmonar con septo interventricular íntegro y estenosis pulmonar crítica entre los meses de enero de 1996 y enero de 2018. Se recogieron y analizaron las características morfológicas del ventrículo derecho, el manejo quirúrgico, la intervención percutánea y la evolución a corto y a largo plazo.
ResultadosCincuenta y un pacientes fueron incluidos. Un total de 9 (17,6%) fallecieron durante el seguimiento. Ninguno de ellos presentaba fístulas arteriales coronarias a ventrículo derecho. La media de seguimiento de los restantes 42 supervivientes fue de 107,81 meses (±53,56).
La clase funcional según la New York Heart Association en la revisión más reciente fue de 1,2. Los supervivientes del grupo de estenosis pulmonar crítica presentaban una clase funcional de 1,1 y los del grupo de atresia pulmonar con tabique interventricular íntegro de 1,6. No hubo diferencias entre los pacientes que presentaban fístulas arteriales coronarias a ventrículo derecho y los que no.
ConclusionesLa presencia de fístulas arteriales coronarias a ventrículo derecho no es una contraindicación para la vía biventricular. Los pacientes con estenosis pulmonar crítica presentan una mejor evolución que los afectos de atresia pulmonar con tabique interventricular íntegro. La estrategia de apertura agresiva y precoz de la válvula pulmonar tiene una buena supervivencia global correlacionada con una buena clase funcional.
Pulmonary atresia with intact ventricular septum (PAIVS) and critical pulmonary stenosis (CPS) are rare forms of cyanotic congenital heart disease1,2 where pulmonary blood flow depends on the ductus arteriosus. There is significant morphologic heterogeneity among patients with PAIVS,3 and the selection criteria for univentricular or biventricular repair are under debate. The right ventricle (RV) morphology, the initial tricuspid valve (TV) z-score4 and the presence of coronary artery to right ventricle fistulae5 and/or right ventricle-dependent coronary circulation6 may influence the decision in favour of the biventricular or the univentricular pathway. However, CPS is different from PAIVS in that the RV and the TV dimensions are close to normal, which makes biventricular repair possible. Historically, some groups have considered the presence of coronary artery to RV fistulae a contraindication for decompression of the right ventricle.7,8
The main treatment goal in the neonatal period is to achieve anterograde flow across the RV outflow tract with the aim to improve systemic arterial oxygenation.9 Both surgical and percutaneous-based procedures1,10–15 have been described in the literature to relieve the RV obstruction and promote RV growth and a greater likelihood of a biventricular repair later in life.16,17
There is no question that the selected approach will have an impact on the quality of life, morbidity and mortality of patients.18 Since PAIVS and CPS are rare congenital heart diseases, most published studies included fewer than 30 patients,19 making it difficult to draw conclusions about treatment strategies.
The aim of this study was to review the medium- and long-term outcomes of PAIVS and CPS patients over 20 years, including patients with coronary artery fistulae, and identify factors that differ between both groups.
Material and methodsWe conducted a retrospective descriptive study in which we identified all patients with a diagnosis of PAIVS or CPS admitted to our neonatal intensive care unit between January 1996 and January 2018.
The study was done in adherence to the Declaration of Helsinki.
The inclusion criteria were diagnosis of PAIVS or CPS, regardless of the size or characteristics of the right ventricle.
We excluded patients with ventricular septal defects, with either discordant atrioventricular and/or ventriculoarterial connections or with other major cardiovascular anomalies.
The study included a total of 51 patients. For each patient, we reviewed the available health records, echocardiograms, angiograms and operative reports. We retrieved data on the following variables from the health records: gestational age, weight, sex, initial intervention offered, complications, need for subsequent additional interventions in survivors and the type of intervention, and further management for biventricular or univentricular circulation. We also collected the age at death and cause of death in patients that did not survive.
We classified patients into severity subgroups (mild, moderate or severe hypoplasia) according the TV z-score,20 partite status, RV outflow tract diameter and presence coronary artery to RV fistulae.21,22
Management in the neonatal periodOnce the diagnosis of PAIVS or CPS was made, the patient was given intravenous prostaglandin E1 on an urgent basis at an initial dose that ranged between 0.005 and 0.1ng/kg/min. The dose was then reduced to the lowest possible that guaranteed an oxygen saturation of around 90%. None of the patients underwent balloon atrial septostomy. Once the newborns were stable enough and pulmonary vascular resistance had decreased below the systemic arterial blood pressure, they were transferred to the catheterisation laboratory.
At the time of the study, the approach for newborns with PAIVS or CPS involved transcatheter balloon-assisted techniques with or without radiofrequency perforation of the pulmonary valve. If the patient required radiofrequency perforation, it was performed with a 2F cable with 5W of energy administered for 1–2s under fluoroscopic guidance. If the perforation was successful, the pulmonary valve was dilated with a balloon measuring 1.2–1.4 times the pulmonary annulus. A procedure was considered successful when it achieved a gradient of less than 30mmHg across the right ventricular outflow tract.
Prostaglandin infusion was maintained for some days after establishment of antegrade pulmonary blood flow if the oxygen saturation remained below 92%. An additional shunt was considered in cases where it was not possible to wean the patient off prostaglandin. If oxygen saturation exceeded 92%, prostaglandin infusion was discontinued.
Ductal stent placement was the first treatment option in cases considered to require an additional shunt. In these patients, we discontinued prostaglandin infusion 4–6h before the procedure. The usual approach for the procedure was through femoral arterial access, with placement of a stent of up to 4mm of diameter across the duct from the aortic side.
In cases where ductal stenting was not possible (newborns with an aneurysm in the ductus or a tortuous ductus, or low-birth-weight newborns), the next option to consider was a systemic-to-pulmonary shunt. The technique used most commonly for this was a right-sided modified Blalock-Taussig shunt (BTS). The location of the shunt was chosen based on anatomic considerations.
We did not exclude performance of this procedure in patients with coronary artery to RV fistulae, and they were also treated as described. The diagnosis of coronary artery fistulae was initially made by means of echocardiography and subsequently confirmed in the catheterisation laboratory. In this group of patients, the pulmonary valve was dilated progressively with balloons of different sizes.
Additional proceduresThe decision whether to perform a biventricular repair, one-and-a-half ventricular repair or univentricular palliation was based on right ventricle function and growth. We considered biventricular repair successful when extrapulmonary flow was not necessary after closure of the atrial septal defect or when there was no systemic desaturation in the absence of right-to-left interatrial shunt as evinced by echocardiography (oxygen saturation>92%). One-and-a-half ventricular repair consisted in the combination of anterograde flow across the tricuspid and pulmonary valves with a superior cavopulmonary anastomosis (Glenn) or a bidirectional shunt across an atrial septal defect in patients that did not undergo Glenn surgery with an oxygen saturation of less than 92%. Univentricular palliation was defined as Glenn surgery with no anterograde flow across the pulmonary valve or total cavopulmonary connection (Fontan).
Statistical analysisWe summarised patient variables using standard descriptive statistics, expressing normally distributed continuous variables as mean±standard deviation (SD), skewed continuous variables as median and range, and categorical variables as absolute and relative frequencies. We assessed differences in patient-dependent variables based on several outcomes of interest using the Mann–Whitney U test, the log-rank test or the Pearson chi-square test as appropriate. We calculated survival rates using the Kaplan–Meier method. We defined statistical significance as established a P-value of less than .05 in any of the tests. The statistical analysis was performed with the software IBM SPSS version 22.
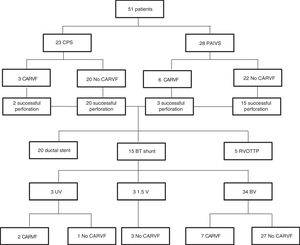
ResultsA total of 51 patients were admitted to our hospital with CPS (23 patients) or PAIVS (28 patients). Of those, 9 (17.6%) had coronary artery to RV fistulae. The diagnosis was prenatal in 39 patients (76.5%). Only 2 of them received a prenatal diagnosis of coronary artery to RV fistulae. Twenty-seven patients (52.9%) were female, and 8 (15.7%) born preterm (at <37 weeks’ gestational age). The incidence of extracardiac anomalies was higher in patients with PAIVS compared to patients with CPS (21.4% vs 4.3%, P=.037). The detected extracardiac anomalies were polycystic kidney disease in 2, intestinal malrotation in 2, pyloric stenosis in 1, ventriculomegaly in 1 and white matter abnormality in 1. Table 1 compares the RV partite status and TV characteristics in patients with PAIVS and patients with CPS.
Demographic and clinical characteristics of the patients.
| Variable | CPS (n=23) | PAIVS (n=28) | P |
|---|---|---|---|
| Female sex | 12 (52.2%) | 15 (53.6%) | NS |
| GA±SD (weeks) | 37.7±3.8 | 38.7±2.0 | NS |
| Preterm birth (GA<37 weeks) | 4 (17.4%) | 4 (14.2%) | NS |
| BW±SD (kg) | 2.85±0.80 | 3.11±0.59 | NS |
| SGA | 3 (13.0%) | 5 (18.5%) | NS |
| Prenatal diagnosis | 15 (65.2%) | 24 (85.7%) | NS |
| Extracardiac malformations | 1 (4.3%) | 6 (21.4%) | <.05 |
| RV partite status | |||
| Tripartite | 19 (82.6%) | 22 (78.6%) | NS |
| Bipartite | 4 (17.4%) | 4 (14.2%) | NS |
| Unipartite | 0 | 2 (7.1%) | <.05 |
| TV z-score | −1.8±0.62 | −2.9±0.85 | <.05 |
| RVOT z-score | −2.65±1.18 | −3.64±1.63 | <.05 |
| MV annulus (mm) | 13 (12–14) | 13 (12–14) | NS |
| TV/MV ratio | 0.64 (0.55–0.71) | 0.86 (0.69–0.8) | <.05 |
| CARVF | 3 (13%) | 6 (21.4%) | NS |
BW, birth weight; CARVF, coronary artery to right ventricle fistula; CPA, critical pulmonary stenosis; GA, gestational age; MV, mitral valve; NS, not significant; PAIVS, pulmonary atresia with intact ventricular septum; RVOT, right ventricular outflow tract; SD, standard deviation; SGA, small for gestational age (BW<10th percentile); TV, tricuspid valve. Statistically significant values are presented in boldface.
The median age of the patients at the time of the first intervention was 8 days (range, 2–20) and the median weight 3.1kg (range, 2.6–3.5). The median age at the time of the first procedure in the catheterisation lab was 6 days (range, 2–18), at the time of ductal stenting 12 days (7–24 days) and at the time of BTS 10 days (6–17 days). Table 1 compares major features between both groups.
Table 2 summarises the procedures performed in the neonatal period. When it came to severity of disease, PAIVS patients required mechanical ventilation longer than CPS patients (3 days [range, 1–56] vs 1 day [range, 1–7]; P<.05). When we took into account the presence of coronary artery to RV fistulae, we did not find any additional differences in postoperative outcomes.
Procedures used in neonatal management and their outcomes.
| Variable | CPS (n=23) | PAIVS (n=28) | P |
|---|---|---|---|
| Balloon valvuloplasty | 22 (95.7%) | 18 (64.2%) | NS |
| Radiofrequency+balloon valvuloplasty | 0 (0%) | 16 (57.1%) | <.05 |
| Ductal stent | 5 (21.7%) | 15 (53.6%) | <.05 |
| Modified BT shunt | 3 (13.1%) | 12 (46.4%) | <.05 |
| RVOTTP | 4 (17.3%) | 1 (3.5%) | <.05 |
| Post-op duration of MV, days (range) | 1 (1–7) | 3 (1–56) | <.05 |
| Length of stay, days (range) | 27 (7–130) | 25 (8–83) | <.05 |
| Mortality | 1 (4.3%) | 8 (28.6%) | <.05 |
BT, Blalock-Taussig; CPS, critical pulmonary stenosis; MV, mechanical ventilation; PAIVS, pulmonary atresia with intact ventricular septum; RVOTTP, right ventricular outflow tract transannular patch.
Two patients (2.8%) died during neonatal management. One had PAIVS and the other CPS. Neither had a coronary artery to RV fistula. One patient developed low cardiac output syndrome after ductal stenting due to circular shunting resulting from pulmonary and tricuspid valve regurgitation and a dysfunctional right ventricle. The patient did not respond to an emergency ductal ligation and developed severe neurologic damage. Another patient, who had an associated diagnosis of CHARGE syndrome, had a cardiac arrest due to ventricular fibrillation during catheterisation.
None of the patients that underwent percutaneous opening of the pulmonary valve suffered RV outflow tract perforation. We did not perform atrial septostomy in any of our patients during the neonatal period.
Long-term outcomesThe median duration of followup from the time of admission in the overall sample was 8.9 years (range, 1–16). A total of 7 patients (13.7%) died during the followup. At the time of this writing, 34 patients (85%) had achieved biventricular circulation, 3 (7.5%) underwent 1.5-ventricular repair successfully, and 3 (7.5%) had univentricular palliation. In the biventricular pathway subset, 10 patients required percutaneous closure of the atrium septal defect. Two patients in the PAIVS survivors group underwent the bidirectional cavopulmonary shunt procedure but have not been scheduled yet for Fontan procedure. Table 3 summarises these results.
End state in treated survivors based on the presence or absence of coronary artery to right ventricle fistulae.
| CARVF (n=9) | No CARVF (n=31) | P | |
|---|---|---|---|
| Univentricular | 2 (22.2%) | 1 (3.2%) | <.05 |
| 1.5-Ventricular | 0 (0%) | 3 (9.6%) | <.05 |
| Biventricular | 7 (77.8%) | 27 (87.1%) | NS |
CARVF, coronary artery to right ventricle fistula; NS, not significant.
When it came to patients with coronary artery to right ventricle fistulae, 7 currently have biventricular circulation and 2 univentricular circulation. There were no deaths in this group. Fig. 1 presents a flow chart of these outcomes. Eight patients achieved biventricular circulation even though the initial percutaneous-based opening of the RV outflow tract was not successful. The mean New York Heart Association functional grading was 1.2 in the patients with biventricular repair, 1.3 in those with 1.5-ventricular repair and 2.0 in those with univentricular palliation. We found no differences between groups when took into consideration the presence or absence of coronary artery to RV fistulae.
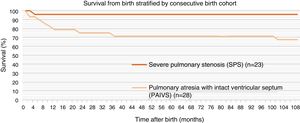
In the entire sample including patients with coronary artery fistulae, the estimated overall survival at 1, 5 and 10 years after the initial hospital admission was 95% for all timepoints in the CPS group and 80%, 70% and 68% in the PAIVS group (Fig. 2).
Lastly, none of the patients in the sample under study experienced complications such as RV rupture during the catheterisation procedure or required balloon atrial septostomy.
DiscussionOutcomes were better in patients with CPS compared to those with PAIVS. Most studies published to date have included patients with CPS and PAIVS in the same category,23,24 but there is enough evidence to consider these heart diseases as separate entities. An aggressive approach with early opening of the pulmonary valve is associated with a better functional class, which in turn allows a biventricular or a 1.5 ventricular definitive repair.
Survival in our overall sample was similar to the survival reported in previous studies.24,25 When we stratified by type of congenital heart defect, we found that the 1-year survival was 95.7% in the CPS group compared to the 80% in the PAIVS group at the same age. As we mentioned above, most of the studies in the literature include patients with CPS and with PAIVS in the same groups, and we think this could explain the considerable differences in mortality between studies.6 We did not see any differences in survival in patients with coronary artery to RV fistulae.
Some authors consider the presence of coronary artery to RV fistulae an absolute contraindication for RV decompression. In light of our results, we believe that this should be carefully reconsidered. If the RV is not decompressed, the course of disease will remain unchanged. This means that oxygen delivery to the myocardium will depend on the perfusion pressure and the oxygen saturation in the RV, neither of which will improve without decompression. First, if the RV is not decompressed, a right-to-left shunt will persist at the atrial level, keeping the oxygen saturation low in the RV. Second, high pressures in the RV will lead to chronic subendocardial ischaemia and eventually to RV failure and low perfusion pressure. This situation inevitably leads to chronic ischaemia and worsening of both systolic and diastolic function in the myocardium. If the RV is not decompressed early in the neonatal period, the infant will soon require a procedure to exclude the RV from the circulation, which eventually leads to cardiac haemodynamics that place the patient in the univentricular pathway. In our series, almost 80% of the patients with coronary artery to RV fistulae achieved biventricular circulation after performance of RV decompression in the neonatal period.
In our opinion, it is crucial to wait for the pulmonary vascular resistance to decrease below the arterial pressure to decompress the RV, usually after the first week of life. In this regard, the patient should be given the minimum possible amount of prostaglandin E1 to guarantee pulmonary flow with a target oxygen saturation of approximately 90%. This will also be beneficial after RV decompression, as the ductus arteriosus will be smaller in size and will not contribute to RV dysfunction through a circular shunt. In these cases where the ductus stays open, if it is large there is a high risk for a circular shunt after RV decompression. This state is difficult to manage in patients with these diseases. In a previously published study, our group demonstrated the usefulness of banding the ductus arteriosus to manage this situation succesfully.26
An atrial shunt is a must in these cardiac patients. A balloon atrial septostomy is rarely necessary during the neonatal period. Previous published studies describe its routine performance. We did not perform a balloon atrial septostomy in any of the patients to prevent the potential development of a circular shunt, as this condition is extremely difficult to manage in the neonatal period. Moreover, an increased right-to-left shunt at this level will contribute to cyanoses and decrease the likelihood of the RV and the pulmonary vascular tree growing.
One of the main reasons for choosing the ductal stent strategy is to reduce the early mortality associated with the BTS, of around 7%.27 It is worth noting that there were no deaths in the BTS group in our sample (0/16), while there were 2 deaths in the ductal stent group (2/20). We think that this may have to do with the neonatal strategy of delaying the opening of the RV outflow tract until the pulmonary vascular resistances start decreasing. Unlike what has been described by other authors,15 once the RV had been opened, our team did not place the ductal stent or performed the BTS in the same surgery, and instead waited for the RV to adjust to the presence of anterograde flow with prostaglandin infusions, with a median age of 12 days at the time of ductal stent and of 8 days at the time of BTS. This may have helped to transition to a balanced circulation between the foetal and the neonatal life, allowing for ductal narrowing and preventing the phenomenon of overcirculation with a low cardiac output and pulmonary overflow that can occur if the BTS or the ductal stent are placed too early. Thus, we think that in these patients is extremely important to wait for the right moment, determined on a case-to-case basis.
Few studies have reported on the clinical outcomes of the 1.5 ventricular repair strategy.28,29,4 In our small sample, we did not find differences in the functional class between the 1.5 and the biventricular repair groups. In all 3 patients that ended with a 1.5-ventricle physiology, the atrium septal defect was closed and the RV showed adequate growth with preserved diastolic function after the surgery. Since the main challenge in patients with borderline RV is to choose between the biventricular or the univentricular pathway for management during the neonatal period, we think that the initial strategy of opening the RV outflow tract and the contribution of an extra source of pulmonary flow may promote RV adaptation, thus rescuing some patients that would have otherwise ended in the univentricular pathway. In our sample, 85% of survivors achieved a biventricular circulation, which is a higher proportion compared to most published case series.15,4
In agreement with prior studies,30 our study found that TV size is a major determinant in achieving a balanced biventricular circulation. We found that patients with CPS had larger TV annulus sizes compared to patients with PAIVS, which we think may account for the greater morbidity in the PAIVS group.
In contrast to other series,7 in our study, neither the patients with CPS nor the patients with PAIVS experienced any catheter-related complications, such as myocardial perforation or cardiac tamponade, probably due to the extensive experience of our cardiology team.
We ought to mention some of the limitations of our study. It was a single-centre retrospective study with a small sample size, which made it difficult to compare outcomes with other studies, as there were only 9 patients with coronary artery to RV fistulae.
Even though predicting the eventual circulation pathway in patients with PAIVS and CPS remains challenging, we advocate for early RV opening during the neonatal period in order to increase the chances of biventricular repair. The neonatal management of these patients should be individualised, and it is necessary to differentiate between CPS and PAIVS, as they have very different outcomes. Moreover, we think that opening the RV and either ductal stent or BTS are safe and effective procedures as long as patients are selected appropriately; it is also important to perform the procedure at the right time, when the pulmonary vascular resistance starts to decrease, in order to help the RV to couple with the anterograde flow.
Conflict of interestThe authors declare no conflict of interest.
Please cite this article as: Vall Camell M, Rodríguez-Fanjul J, Bautista Rodríguez C, Pradda FH, Caffarena-Calvar JM, Sánchez-de-Toledo J, et al. Manejo percutáneo de atresia pulmonar con tabique interventricular íntegro y estenosis pulmonar crítica. An Pediatr (Barc). 2019;91:336–343.
Previous presentations: This study was presented at the 6th Congress of the European Academy of Paediatric Societies (EAPS), October 2016, Geneva, Switzerland; and the 7th World Congress of Pediatric Cardiology and Cardiac Surgery (WCPCCS), July 2017, Barcelona, Spain.